Abstract
Background Cognitive decline is a significant concern for stroke survivors, affecting their quality of life and increasing their burden on the healthcare system. DL-3-n-butylphthalide (butylphthalide) has shown efficacy in the short-term treatment of various cognitive impairments. This study evaluated the efficacy of butylphthalide in preventing cognitive decline over a 12-month period in patients with ischaemic stroke.
Methods This prospective following-up study involved patients newly diagnosed with ischaemic stroke between 1 month and 6 months after stroke onset and not in the acute phase. Patients were assigned to either the butylphthalide or control group. Cognitive function was assessed using the mini-mental state examination (MMSE) at baseline and at the 12-month follow-up. Statistical analyses included t-tests, χ2 tests and multivariate regression analyses.
Results Butylphthalide was negatively associated with the MMSE D-value (β=−0.122; 95% CI −1.932 to −0.298; p=0.003) and the MMSE D-value percentage (β=−0.117; 95% CI −0.057 to −0.011; p=0.004). A multivariate analysis indicated that butylphthalide treatment was negatively associated with both changes in orientation and language score. Additionally, the incidence of cognitive decline was significantly lower in the butylphthalide group (OR, 0.612; p=0.020) than the control group. An age of ≥60 years and lower educational level were identified as risk factors for lower cognitive score and cognitive decline.
Conclusion This study demonstrated that butylphthalide is effective in preventing cognitive decline in patients with ischaemic stroke. These findings have significant implications for clinical practice, suggesting that butylphthalide could be incorporated into standard post-stroke care regimens to improve patient outcomes and reduce the healthcare burden. Additional multicentre double-blind trials are recommended to confirm these results in diverse populations.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Butylphthalide can improve cognitive decline in patients with ischaemic stroke. However, it is unclear whether butylphthalide can prevent cognitive decline in ischaemic stroke survivors with normal cognitive levels.
WHAT THIS STUDY ADDS
We found that after 12 months of follow-up, both the cognitive scores of patients in the butylphthalide group and the control group decreased, but the trend of decrease was smaller in the butylphthalide group.
Further analysis revealed that the incidence of cognitive decline events was 16.5% in the butylphthalide group, significantly lower than the control group.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study enhances the neuroprotective effect of butylphthalide, which can complement existing treatment methods and provide a more comprehensive strategy for managing the multifaceted effects of stroke.
Introduction
By 2050, an estimated 40 million elderly individuals, worldwide, are expected to suffer from cognitive impairment.1 At the end of 2018, the prevalence of mild cognitive impairment among people in China who were at least 60 years old was 15.5%, or approximately 38.77 million individuals.2 Stroke is a common risk factor for cognitive impairment, with approximately 38% of patients experiencing cognitive impairment within 12 months post-stroke.3 Long-term follow-up studies of stroke survivors showed a 35.6% prevalence of cognitive impairment 5 years post-stroke, indicating a long-term risk of cognitive decline among stroke survivors.4 Among stroke survivors, cognitive impairment increases disability and dependency, burdening them, their caregivers and healthcare systems.5–8
Butylphthalide, a compound derived from celery seeds, has shown efficacy in treating various pathological stages of acute ischaemic stroke.9–11 Its effectiveness in improving cognitive function has been recognised in patients with vascular cognitive impairment, post-stroke cognitive impairment, Parkinson disease, cognitive impairment and Alzheimer disease.12–16 Previous clinical trials have primarily focused on treating post-stroke cognitive impairment rather than preventing cognitive decline in patients with ischaemic stroke who have not yet developed cognitive impairment.17 However, the effectiveness of butylphthalide on preventing post-stroke cognitive impairment in patients who have experienced ischaemic stroke has not been reported. Therefore, this study investigated the effectiveness of butylphthalide at preventing cognitive dysfunction in patients 12 months after ischaemic stroke.
Methods
Trail design
This prospective following-up study involved patients newly diagnosed with ischaemic stroke between 1 month and 6 months after stroke onset and not in the acute phase. Participants were recruited from community in staged cluster sampling at a 1:1 ratio. In the first stage, subjects meeting the inclusion criteria would be recruited as the butylphthalide treatment group from April to July 2021, and in the second stage, subjects meeting the inclusion criteria would be recruited as the control group from October 2021 to March 2022. All patients were followed up over a 12-month period to observe the effects of butylphthalide on cognitive decline after ischaemic stroke. The trial design adhered to the Declaration of Helsinki and was approved by the Ethics Committee of Tianjin Medical University General Hospital. Written informed consent was obtained from all participants or their legal representatives.
Study population
The participants were males and females, 18 years old or older, residing in Tianjin who were new diagnosed with non-cardiogenic ischaemic stroke via MRI and who were capable of self-care or who had a direct caregiver. The exclusion criteria included haemorrhagic stroke, recurrent stroke, malignant tumours, coagulation disorders, pregnancy, participation in other clinical trials, pre-existing dementia, severe aphasia or significant liver/kidney dysfunction.
Intervention
All patients received standardised conventional treatment, including anti-platelet, anti-hypertensive, anti-diabetic and/or lipid-lowering medications. Patients in the control group continued their conventional treatment, whereas those patients in the butylphthalide group plus received butylphthalide (0.2 g each time, three times daily) for 12 months.
Data collection at baseline
All participants were interviewed face-to-face at the baseline. Demographic characteristics (including age, sex and educational level), previous familial disease histories (including hypertension, diabetes, coronary heart disease (CHD) and stroke), medication use, individual lifestyle characteristics (including smoking, alcohol consumption and physical exercise) and modified rankin scale (mRS) were collected during the baseline interviews. Blood samples were taken from all participants following 12-hour fasts and were tested for serum fasting blood glucose, total cholesterol, triglyceride, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol and homocysteine levels.
Follow-up
Patients underwent follow-up visits at 3, 6, 9 and 12 months post-treatment to assess risk factor control, adverse reactions, endpoint events and treatment adherence. Endpoint events included new ischaemic strokes, acute coronary syndrome, transient ischaemic attacks, peripheral vascular disease, haemorrhagic strokes and all-cause mortality.
Evaluation of cognitive impairment and outcomes
All participants were evaluated using the mini-mental state examination (MMSE) at baseline and at 12 months. MMSE includes five parts including orientation, registration, attention and calculation, recall and language. Cognitive impairment was defined based on the Chinese Guidelines for the Diagnosis and Treatment of Vascular Cognitive Impairment-2019.18 The cognitive impairment was defined according to MMSE ≤17 for patients without formal education, ≤ 19 for those with 1–6 years of education and ≤24 for those with more than 6 years of education. The primary outcome was the D-value (baseline score minus score after 12 months of treatment) and D-value percentage in MMSE score and cognitive domain scores before and after treatment. The secondary outcome was the incidence of cognitive decline. According to the previous studies, cognitive decline was defined as MMSE D-value ≥ 3.19 20
Statistical analysis
Continuous variables were expressed as means and SD; categorical variables were expressed as numbers with frequencies. Between-group differences were compared using χ2 tests for categorical variables and t-tests or analysis of variance for continuous variables. Variables with p<0.05 in univariate analysis are included in the relevant multivariate analysis. Multivariate analyses were conducted using linear regression for MMSE score changes and logistic regression for the incidence of cognitive decline. The results are presented as adjusted OR and 95% CIs, reflecting associations observed within following-up. SPSS (V.25.0; IBM, Armonk, NY, USA) was used for all statistical analyses; two-tailed P values<0.05 were considered statistically significant.
Results
In the first stage, 596 patients meeting the inclusion criteria were recruited as the butylphthalide treatment group from April to July 2021, and in the second stage, 594 patients meeting the inclusion criteria were recruited as the control group from October 2021 to March 2022. During the follow-up visit, 40 patients withdrew from the butylphthalide group because of the unpleasant smell of celery, mild nausea and decreased appetite, mild allergy and dizziness; 21 patients were lost to follow-up; and 3 patients died. In the control group, 2 patients had a dizziness, 68 patients were lost to follow-up and 4 patients died. 532 participants in the butylphthalide group and 520 participants in the control group completed the follow-up evaluation after 12 months of treatment. Excluding 209 patients with cognitive impairment at baseline and 238 patients who were not assessed for their baseline and/or 12-month MMSE, a total of 605 patients (309 in the butylphthalide group and 296 in the control group) were included in the analysis of this study (figure 1).
Flow chart. Participant flow for the study of butylphthalide treatment and cognitive decline in ischaemic stroke survivors.
Baseline characteristics
The baseline characteristics of the study participants are summarised in table 1. In the butylphthalide group, 68.6% were men, and 31.4% were women. The mean age was 61.43 years overall. The average educational level was 7.27 years overall. 5.8% had not received formal education, 36.6% had 1–6 years, and 57.6% had >6 years. In the control group, 72.3% were men, and 27.7% were women. The mean age was 62.17 years overall. The average educational level was 7.06 years overall. In addition, 5.7% had not received formal education, 40.9% had 1–6 years, and 53.4% had >6 years. The prevalence rates of hypertension, diabetes and CHD in the butylphthalide group were 78.3 %, 32.0% and 12.9%. In the control group, the prevalence rates of hypertension, diabetes and CHD were 71.3 %, 28.7% and 10.5%.
Baseline characteristics of the patients
Primary outcome: the difference of cognitive score between before and after 12-month butylphthalide treatment
Influence of butylphthalide on mini-mental state examination (MMSE) score
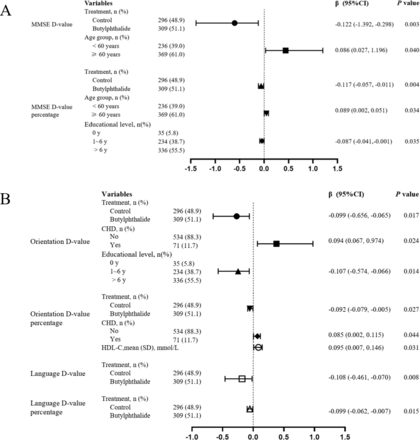
The multivariate analysis was adjusted for treatment group, age group, education level and HDL-C, which were associated factors of MMSE D-value and D-value percentage, in the univariate analysis (p<0.05; online supplement table 1). The mean difference in MMSE scores (D-value) was 0.21±2.91 in the butylphthalide group compared with 1.13±3.93 in the control group (p=0.001). Similarly, the MMSE D-value percentage was significantly lower in the butylphthalide group (0.01±0.12) compared with the control group (0.04±0.17; p=0.002; table 2). Figure 2A showed that butylphthalide treatment was independently associated with smaller MMSE D-value (β=−0.122; 95% CI −1.392 to −0.298; p=0.003) and D-value percentage (β=−0.117; 95% CI −0.057 to −0.011; p=0.004). In addition, age was a significant factor affecting MMSE score, with patients aged 60 years and older experiencing greater declines in MMSE D-value (β=0.086; 95% CI 0.027 to 1.196, p=0.040) and D-value percentage (β=0.089; 95% CI 0.002 to 0.051; p=0.034).
Supplementary data
Multivariate analysis of MMSE score, orientation score and language ability score before and after treatment with butylphthalein. The forest plots displayed the results of multivariate analysis. (A) MMSE D-value and D-value percentage; (B) D-value and D-value percentage of orientation and language. The horizontal bars represent 95% CIs. Different shapes of the horizontal bars represent different influencing factors; β>0 indicates a positive correlation between the independent variable and the dependent variable, while β<0 indicates a negative correlation. CHD, coronary heart disease; HDL-C, high-density lipoprotein cholesterol; MMSE, mini-mental state examination.
Differences in MMSE scores and cognitive domain scores in patients before and after treatment with butylphthalide
Influence of butylphthalide on cognitive domain score
Further analysis of the cognitive domain scores revealed that butylphthalide had a favourable effect on the orientation and language domain results within the MMSE. The mean orientation D-value was 0.02±1.56 for the butylphthalide group vs 0.40±2.07 for the control group (p=0.010). For the language domain, the mean D-value was 0.04±1.13 for the butylphthalide group compared with 0.31±1.32 for the control group (p=0.008). There were no significant differences between the groups in other cognitive domain (table 2). In univariate analysis (online supplement table 2), the factors related to the Orientation D-value and D-value percentage included treatment group, age group, education level, hypertension, diabetes, CHD, baseline systolic blood pressure (SBP) level and HDL-C level (p<0.05). Similarly, a univariate analysis was conducted on the Language D-value and D-value percentage (online supplement table 3), and the only relevant factor was treatment group. Multivariate analysis incorporated relevant factors from univariate analysis. Compared with the control group, the butylphthalide group showed a smaller D-value in orientation (β=−0.099; 95% CI −0.656 to −0.065; p=0.008) and language ability (β=−0.108; 95% CI −0.461 to −0.070; p=0.015; figure 2B).
Secondary outcome: incidence of cognitive decline
Multivariate analysis confirmed that butylphthalide treatment was associated with a lower risk of cognitive decline (OR, 0.612; 95% CI 0.405 to 0.925; p=0.020; figure 3), after adjusted the related factors including treatment group, gender, age group, educational level, smoking status, alcohol use and HDL-C in univariate analysis (p<0.05; table 3). The incidence of cognitive decline at 12 months showed that 51 patients (16.5%) in the butylphthalide group developed cognitive decline compared with 71 patients (24.0%) in the control group (p=0.022). Educational level also played a critical role, with higher educational attainment being associated with lower cognitive decline risk (OR, 0.704; 95% CI 0.504 to 0.984; p=0.040; figure 3).
Multivariate analysis of cognitive decline. Treatment group and educational level played a important role in the incidence of cognitive decline. The horizontal bars represent 95% CIs. OR >1 indicates a positive correlation between the independent variable and the dependent variable, while risk ratio <1 indicates a negative correlation.
Univariate analysis of the risk of cognitive decline
Discussion
This study is the first to report the long-term effects of butylphthalide on cognitive function in patients with ischaemic stroke, particularly its role in preventing cognitive decline 12 months post-stroke. This study provides promising findings that butylphthalide may be effective in preventing cognitive decline in patients with ischaemic stroke. These findings highlight the potential of butylphthalide as a therapeutic intervention for improving cognitive outcomes in stroke survivors.
Brain health, as part of optimising quality of life and controlling global healthcare costs, is closely related to the prevention of cognitive impairment.21 Cognitive impairment is characterised by acquired and sustained cognitive impairment, which lead to decreases in the patient’s daily work ability and behavioural changes.22 Currently recommended drugs for improving cognitive impairment include cholinesterase inhibitors (donepezil, rivastigmine and galantamine), N-methyl-D-aspartate receptor antagonists (memantine) and other drugs (extracts of Ginkgo biloba leaves and Salvia miltiorrhiza); however, their effects are unclear.
Butylphthalide entered the Chinese market as an anti-ischemia drug in 2002. Later, animal experiments showed that butylphthalide could regulate the expression of NR2B and synaptophysin in the hippocampus of elderly rats after chronic cerebral hypoperfusion, increase brain acetylcholine levels and improve learning and memory.23 A subsequent clinical trial indicated that the administration of butylphthalide to 281 adults with subcortical vascular cognitive impairment without dementia during a 6-month treatment period was safe and effective for improving cognitive and overall function.13 This may be related to the ability of butylphthalide to reduce neuroinflammation, oxidative stress and synaptic plasticity in the hippocampus.24–26
In contrast to previous studies, our study focused more on whether butylphthalide could prevent cognitive decline in patients with ischaemic stroke rather than on its therapeutic effects. We found that butylphthalide reduced the incidence of cognitive decline by 7.5%, compared with the control group, after 12 months of treatment.
The MMSE is an effective means of evaluating cognitive function, and previous studies have used the MMSE score to evaluate changes in cognitive function caused by butylphthalide. A meta-analysis of seven clinical studies that used the MMSE to assess the effect of butylphthalide on cognitive function showed that treatment with butylphthalide improved, to varying degrees, the scores of patients with post-stroke cognitive impairment.14 This study describes the changes in MMSE scores based on the MMSE D-value and percentage of MMSE D-value. Although both the butylphthalide and control groups showed decreased MMSE scores after 12 months of treatment, the extent of the decrease was lower in the butylphthalide group.
In addition to the different treatments received, age and educational level were found to play important roles in the progression of cognitive decline, consistent with previous research findings.27 Lower educational level is associated with increased cognitive impairment.28 The possible reasons why people with higher levels of education have better cognitive function are greater cognitive reserve and better indirect benefits (free from the influence of risk factors of cognitive impairment such as hypertension, diabetes and cardiovascular factors).29
This study had certain limitations. First, the educational level of the study population was determined using a survey and was stratified according to educational levels. The educational level of the study participants was generally low, which has been shown to be a risk factor for cognitive impairment.30 This suggests a potential tendency for cognitive dysfunction in this population. Therefore, further research is required to investigate the efficacy of butylphthalide in populations with different levels of education. The second limitation of the study is the use of phased cluster sampling rather than complete randomisation. While this method allowed for practical recruitment in a community-based setting, it may introduce selection bias. However, multivariate analyses were conducted to adjust for baseline differences between the groups, minimising the impact of potential confounders. Third, the MMSE scores were analysed based on the total score, and individual scores could not be considered to correspond to cognitive performance. Further examination in the field of neuropsychology is needed to investigate the impact of butylphthalide on various cognitive domains in patients with stroke. Finally, the limitation of this study is the absence of certain baseline characteristics that could significantly impact post-stroke cognitive function, such as the premorbid mRS, National Institutes of Health Stroke Scale (NIHSS) at admission and discharge and the 3-month mRS. However, this study specifically targeted recovery-phase stroke patients—individuals whose strokes occurred more than 1 month but less than 6 months prior to the study, all of whom were community residents. As such, the acute-phase clinical data, including NIHSS scores and premorbid mRS, were not available or deemed necessary for this particular study population. The absence of these variables could limit the precision of the study’s findings, particularly in accounting for stroke severity in the early phase. Without NIHSS and mRS data, we may be unable to fully adjust for the potential variability in stroke severity among participants. This might introduce some degree of bias or confounding. Future studies could improve on this limitation by incorporating more detailed clinical data, including NIHSS and mRS scores from the acute phase.
This study suggests that butylphthalide is effective at reducing cognitive score decrease and the incidence of cognitive decline over a 12-month period in patients with ischaemic stroke. These results reinforce the neuroprotective benefits of butylphthalide against cognitive impairment. Butylphthalide can effectively mitigate cognitive deficits, enhance daily functioning, increase independence and improve the overall quality of life of patients. Moreover, butylphthalide could potentially reduce long-term healthcare needs and associated costs for stroke survivors, including decreasing the need for the intensive care and support services that are often required for patients with significant cognitive impairment. These benefits could complement existing treatments and provide a more comprehensive strategy for managing the multifaceted impacts of stroke.
Data availability statement
The data supporting the findings of this study are available upon reasonable request. Deidentified participant data, including clinical assessments, neurological outcomes, and other relevant measurements, can be obtained from the corresponding author Jinghua Wang. Data will be made available to qualified researchers upon submission of a request that includes a clear research plan, which outlines how the data will be used in compliance with ethical guidelines and relevant privacy protections. Protocols and statistical analysis plans are also available upon request. The reuse of these data is permitted solely for non-commercial research purposes, and any publications resulting from the use of these data must acknowledge the original study. The authors reserve the right to assess requests and provide data based on applicable institutional and ethical guidelines.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants. The trial design adhered to the Declaration of Helsinki and was approved by the Ethics Committee of Tianjin Medical University General Hospital (contract number: IRB2020-YX-056-02). Participants gave informed consent to participate in the study before taking part.
Footnotes
Contributors YL, JW, XN were involved in conception and design and data interpretation for this article. QH, XZ, YS, FT, HW, DW, XL, YW, JT and LW were involved in data collection, case diagnosis and confirmation for this article. QH, XZ and YS were involved in manuscript drafting. JW was involved in data analysis for this article. YL, JW and XN were involved critical review in for this article. YL is the guarantor.
Funding This study was funded by Tianjin Municipal Health Commission (TJWJ2023QN115)
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer-reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.