Abstract
Background MLC1501, consisting of four herbs, that is, Radix Astragali, Radix Angelicae sinensis, Rhizoma Chuanxiong, Radix Polygalae, has the same pharmacological properties as its precursors MLC601 and MLC901 which contain extracts of nine herbs and showed neuroprotective, anti-inflammatory and neurorestorative properties in non-clinical models, as well as clinical benefits in improving functional and neurological recovery after brain injuries.
Aims To determine the efficacy of MLC1501 on motor recovery as measured by Fugl-Meyer motor Assessment (FMA) total score at 24 weeks in patients with ischaemic stroke (IS).
Design A total of 300 patients aged >18 years, diagnosed with IS in the prior 2–10 days, with National Institute of Health Stroke Scale (NIHSS) total score of 8–18 and a combined score of ≥3 on NIHSS motor items 5A, 5B, 6A and/or 6B, will be randomised in a 1:1:1 ratio to receive oral placebo, MLC1501 low dose or MLC1501 high dose for 6 months. The study is governed by a Steering Committee. An independent Data Monitoring Committee oversees patient safety.
Outcomes The primary outcome is mean change from baseline in FMA total score at 24 weeks. Efficacy outcomes evaluated in person at baseline, 12 weeks and 24 weeks include the FMA (total, upper extremity and lower extremity motor scores), modified Rankin Scale (mRS), Patient-Reported Outcomes Measurement Information System–Global Health (PROMIS-10) and NIHSS. Additionally, telephone assessment at week 4 includes the simplified mRS and PROMIS-10.
Safety will be evaluated by standard assessments and occurrence of adverse events over the duration of the study.
Discussion Interventions that enhance recovery beyond the acute period of stroke are needed. MLC1501 has a good safety profile as well as potential to be a treatment for recovery after brain injury. The results of this study will provide objective level B evidence on the efficacy of MLC1501 on long-term recovery and safety of 24 weeks of treatment among patients with IS.
Trial registration number NCT05289947.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Many patients do not receive time-sensitive acute stroke therapies for various reasons. MLC1501 is a simplified formulation of MLC601 and MLC901 that have demonstrated neuroprotective, anti-inflammatory and neurorestorative properties.
WHAT THIS STUDY ADDS
The MLC1501 study Assessing Efficacy in post-STrOke Subjects with mOtor deficits (MAESTOSO) study will provide objective level B evidence on the efficacy and safety of MLC1501 on long-term motor recovery among patients with ischaemic stroke.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
MAESTOSO has the potential to contribute to the scarce research and few available therapies for stroke recovery, thereby reducing overall disease burden.
Background
MLC601 (NeuroAiD) and MLC901 (NeuroAiD II), both containing extracts from nine herbs, have been shown to demonstrate neuroprotective, anti-inflammatory and neurorestorative properties in non-clinical animal and cellular models of focal and global cerebral ischaemia.1–7 Clinical trials of MLC601/MLC901 and systematic reviews of those trials showed benefit in improving functional and neurological recovery in patients with ischaemic stroke.8–23
As part of further drug development, a simplified formulation (MLC1501) would have the advantages of reduced variability, reduced risk of contamination, avoidance of unnecessary animal slaughter, reduced daily number of capsules to improve compliance and lower cost. Literature reviews and a series of in vitro and in vivo experiments were performed to assess the effects of the different herbs in the parent formulation against neuronal degeneration and cerebral ischaemia. The results indicated that the combination of herbs that included MLC1501, that is, Radix Astragali, Rhizoma Chuanxiong, Radix Angelicae sinensis and Radix Polygalae, was most efficient for neuroprotection and neurorestoration. MLC1501 was shown to increase neurite outgrowth and proliferation and has neuroprotective effects as indicated by reduced lactate dehydrogenase release, smaller infarct volume and activation of ATP-dependent potassium channels comparable to that of the precursors (unpublished reports on file).
Furthermore, with only four ingredients, MLC1501 is expected to have a comparable or even better efficacy-to-safety profile than MLC601 and MLC901.8–26 The safety of MLC1501 was demonstrated in two phase I studies on healthy subjects in an investigational new drug application under the botanical drug framework of the US Food and Drug Administration.27
In the dose-escalation study, MLC1501 was shown to be safe and well tolerated. The maximum tolerated dose, defined as the highest dose of a drug or treatment that does not cause unacceptable side effects, was not identified up to a dose of 4000 mg/day in the study, equivalent to extracts from 10.4 g of raw Radix Astragali and 2.08 g each of raw Rhizoma Chuanxiong, Radix Angelicae sinensis and Radix Polygalae daily. MLC1501 was well tolerated with no dose–response observed for treatment emergent adverse events (TEAE), which were all mild and none of which led to premature discontinuation of the study. Headache was the most common TEAE and the only one that was considered as possibly related to study drug by the investigator. No deaths or serious adverse events (SAEs) were reported.
In a drug–drug interaction (DDI) study, the safety and tolerability of MLC1501 was confirmed when given alone or in combination with other drugs in healthy subjects. Two separate cohorts were administered cocktails of drugs acting as sensitive clinical probe substrates of cytochrome P450 isoenzymes or transporters before and after intake of high-dose MLC1501 (4000 mg/day). Results showed no significant adverse event or risk of pharmacokinetic DDI, except for a weak (20%) decrease in exposure of metformin, a sensitive substrate of renal organic cation transporter 2 (OCT2) and multidrug toxin extrusion proteins (MATE1, MATE2-K).
Proving the clinical efficacy and safety of MLC1501 on long-term functional and neurological recovery in patients suffering from ischaemic stroke will fill a therapeutic gap and help reduce the overall burden of stroke.
Trial objectives
The primary objective of the study is to determine the efficacy of MLC1501 compared with placebo in motor recovery as measured by the Fugl-Meyer motor Assessment (FMA) total score at 24 weeks in patients with stroke. Secondary objectives are (1) to assess the safety of MLC1501 in patients with stroke, and (2) to determine the efficacy of MLC1501 compared with placebo in terms of (a) mean change from baseline in FMA total score at 12 weeks, (b) mean change from baseline in FMA upper and lower extremity scores at 12 and 24 weeks, (c) proportion of patients with modified Rankin Scale (mRS) score (0–1 vs 2–6) at 4, 12 and 24 weeks, (d) mean change from baseline in Patient-Reported Outcomes Measurement Information System–Global Health (PROMIS-10) score at 4, 12 and 24 weeks and (e) mean change from baseline in National Institute of Health Stroke Scale (NIHSS) score at 12 and 24 weeks.
Methods
Design and study population
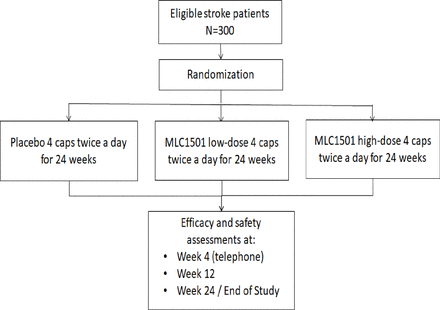
This is a multicentre, randomised, double-blind, placebo-controlled study of MLC1501 in patients with stroke. Box 1 enumerates the inclusion and exclusion criteria. A total of 300 eligible patients will be allocated to receive MLC1501 high dose, MLC1501 low dose or matching placebo for 24 weeks (figure 1).
Schematic diagram of trial design.
Eligibility criteria
Inclusion criteria
Male or female.
≥18 years old or legal age as per country requirement.
Diagnosed with acute ischaemic stroke with compatible brain imaging findings between 2 and 10 days (inclusive) prior to inclusion.
National Institute of Health Stroke Scale (NIHSS) total score of 8–18 (inclusive) at the time of inclusion with a score of at least 3 on the NIHSS motor items 5A or 5B and/or 6A or 6B.
A candidate for active rehabilitation in the opinion of the treating physician.
Able to comply with the requirements of the protocol and provide written informed consent before any study-specific procedure is performed (consent may also be provided by patient’s legal representative if applicable and allowed by local regulatory requirements).
Exclusion criteria
Prestroke modified Rankin Scale score of >1.
Contraindication to any of the study procedures.
Patients who became medically unstable within 24 hours after intravenous or endovascular thrombolysis or thrombectomy.
Intake of any herbal or traditional medicine within the past 30 days.
Participation in another investigational drug or device trial within the past 30 days.
Intake of warfarin in the past 1 week or expected to be on warfarin while in the study.
Women who are pregnant or wish to continue breastfeeding while in the study. Women of childbearing potential may be included if they agree to strict abstinence or use of effective contraception, except systemically acting hormonal contraceptives. Hormone replacement therapy in menopausal/postmenopausal or surgically sterilised women is also not allowed while in the study.
Any known food allergy or hypersensitivity to Astragalus membranaceus, Ligusticum chuanxiong, Polygala tenuifolia, Angelica sinensis or members of the Fabaceae/Leguminosae family (eg, legume, pea, bean), Polygalaceae family (eg, milkwort, snakeroot), Apiaceae/Umbelliferae family (eg, anise, caraway, carrot, celery, dill, parsley, parsnip) or Quillaja bark (soapbark).
Evidence of other significant ischaemic brain lesion which could affect long-term function or disability.
Evidence of advanced or serious medical condition that would affect study assessment and follow-up, such as cancer, renal failure, liver cirrhosis, dementia or psychosis.
Any other medical or psychiatric or cognitive condition which, in the study investigator’s opinion, may jeopardise the patient by his/her participation in this study, may hamper his/her ability to complete procedures required in the study, affect study assessment and follow-up or affect the validity of the study results.
Randomisation and blinding
Eligible participants will be randomised centrally in a 1:1:1 ratio to one of three treatment arms: placebo, MLC1501 low dose or MLC1501 high dose, stratified according to country, NIHSS (8–12, 13–18) and whether subject received either intravenous or endovascular thrombolysis/thrombectomy or not. Investigators, treating physicians, qualified outcome assessors and all other staff involved in the clinical care of the patient as well as the patient themselves will be blinded to treatment allocation. Treatment allocation will be concealed until the end of study, unless unblinding of individual patient treatment allocation is specifically required by the investigator to make appropriate decisions on clinical management or by the Data Monitoring Committee (DMC) during safety reviews.
Study treatment
Each 500 mg MLC1501 high-dose capsule contains approximately 267.7 mg of botanical extract from 2.08 g of raw herbs of Radix Astragali, Rhizoma Chuanxiong, Radix Angelicae sinensis and Radix Polygalae at a ratio of 5:1:1:1, while MLC1501 low dose contains approximately 133.85 mg of extract from 1.04 g of raw herbs per capsule. The placebo capsules match active treatments in colour, taste and odour. Capsule shells are made of hypromellose, size 0, with light blue cap and dark blue body colours. The study treatment capsules are packaged in medicinal high-density polyethylene bottles that are induction sealed to provide additional protection from moisture and closed with a child-resistant screw cap. Each bottle contains 125 capsules and must be stored in an environment not exceeding 30°C.
Study treatments are to be taken orally at a dose of four capsules two times per day for 24 weeks, at least 30 min after a meal. The first dose is to be administered on the day of randomisation. If the subject is unable to swallow and has an enteral feeding tube, for example, nasogastric tube or gastrostomy tube, the capsules may be opened, dispersed in approximately 30 mL of water and administered through the feeding tube followed by water flushing.
All participants will receive standard stroke care and treatment of associated underlying medical conditions and vascular risk factors, including but not limited to antithrombotic therapies, antihypertensive, antidiabetes and lipid-lowering medications, and appropriate rehabilitation.
Intake, either enterally or parenterally, of ‘neuroprotective’ or ‘nootropic’ products which are not standard or not within the national guidelines for treatment of stroke, and/or systemically acting hormonal contraceptives and hormonal replacement therapy are not allowed while in the study. Subjects who subsequently would require anticoagulation after inclusion into the study should consider newer oral (antithrombin or antifactor Xa) anticoagulants. If warfarin is indicated, close monitoring of the international normalised ratio is recommended.
Trial outcomes and endpoints
Table 1 shows the schedule of study procedures including efficacy and safety assessments in the study. The primary endpoint is the mean change from baseline in FMA total score at 24 weeks (±7 days). Efficacy outcomes to be evaluated in person at baseline, 12 weeks (±7 days) and 24 weeks (±7 days) include the FMA (total, upper extremity and lower extremity motor scores), mRS, PROMIS-10 and NIHSS. Additionally, telephone assessment at week 4 (±5 days) includes the simplified mRS and PROMIS-10 questionnaires.
Schedule of study procedures
The safety endpoints will be evaluated by standard safety assessments including physical examination, laboratory testing, ECG and ascertainment of occurrence of adverse events over the duration of the study.
Sample size estimation
The sample size was estimated based on data obtained from previous clinical studies of MLC601 (precursor product of MLC1501) in patients with non-acute ischaemic stroke using FMA score as an endpoint.24 To detect a 10-point difference in mean change from baseline in FMA total score at 24 weeks between placebo and each active treatment arm and assuming an SD of 20, a sample size of 252 patients (84 per group) is required to achieve 90% power at 5% (two-sided) level of significance. In order to account for possible dropouts, 300 patients (100 per group) will be recruited in the study. Randomised patients who are not dosed may be replaced.
Statistical analyses
The primary efficacy analysis will be performed using the intention-to-treat population that includes all randomised patients regardless whether they received treatment or not. Per-protocol analysis will include patients without any significant protocol deviation including compliance to study treatment of ≥80%.
The primary hypothesis to be tested is whether the mean change from baseline in FMA total score at 24 weeks differs from placebo, either for the high-dose group and/or for the low-dose group of MLC1501. The comparison between treatment groups (MLC1501 high dose vs placebo and MLC1501 low dose vs placebo) will be performed using a linear mixed effects model with repeated measurements. In order to maintain the overall type I error at 5% for the primary efficacy analysis, a gatekeeping strategy (hierarchical testing) will be used. The model will include change from baseline in FMA total score at each postbaseline time point simultaneously as a response variable, and baseline FMA total score, randomisation stratification factors (country, NIHSS (8–12 vs 13–18) and received either intravenous or endovascular thrombolysis/thrombectomy (no vs yes)), visit (week 24 vs week 12), treatments (MLC1501 high dose vs placebo, and MLC1501 low dose vs placebo) and interactions between treatments and visits as fixed effects. In addition, the model will include subject-specific intercepts as random effects. The least squares (LS) means for each treatment, LS-means difference (MLC1501 high dose–placebo, and MLC1501 low dose–placebo), corresponding 95% CIs and p values will be reported.
Secondary analyses will include the continuous secondary endpoints (ie, FMA total score at 12 weeks, upper extremity and lower extremity motor scores at 12 and 24 weeks, NIHSS score at 12 and 24 weeks and PROMIS-10 score at 4, 12 and 24 weeks) and will be analysed similarly to the primary endpoint. The mRS score at 4, 12 and 24 weeks will be classified as binary variable (0–1 vs 2–6) and analysed by generalised linear mixed effects model with repeated measurements using binomial distribution and logit link function.
All subjects receiving at least one dose of study drug will be included in the safety analyses. All TEAEs and SAEs will be summarised using frequency and percentage of subjects who experienced them by highest severity and relationship with study treatment. The laboratory, vital signs and ECG parameters will be analysed by descriptive statistics.
Ethical considerations
The trial will be conducted in accordance with the clinical trial protocol and in compliance with Good Clinical Practice (GCP) and applicable regulatory requirements. Necessary approvals from ethics committees (EC) or institutional review boards (IRB) will be obtained prior to study initiation at each site. Before any study-specific procedure, the appropriate informed consent will be obtained. Only approved and current informed consent forms will be used and signed by the patient or patient’s legally acceptable representative before inclusion of the patient in the study and before any study-related procedure is performed. Patients will have the right to withdraw from the study at any time and for any reason without prejudice to her or his future medical care.
Any protocol amendments will be submitted to the EC or IRB for review and approval. No amendment may be implemented until approval for such amendment from the EC or IRB is received, unless the amendment is considered administrative in nature or is necessary to remove or reduce any potential study-related risk to the patient based on new information.
All data and information collected in the trial will be anonymised and identified only by the study patient ID number.
Study organisation
A Trial Steering Committee (TSC) provides oversight for the trial and advises the sponsor on all aspects of the trial. The TSC remains blinded to patient treatment allocation throughout the trial.
A DMC, independent of the investigators and the sponsor, regularly monitors the safety of patients by reviewing the blinded study data, as they accumulate. The DMC reports its recommendations to the TSC.
Study sites are selected based on feasibility and their capability of conducting and completing the study.
Quality control and assurance
The sponsor will be responsible for implementing and maintaining quality assurance, quality control and overall quality integrity in the trial. The investigators will ensure the accuracy, completeness and timeliness of data recording to allow appropriate data queries and reporting.
To reduce inter-rater variability, assessors of clinical outcomes, that is, FMA, mRS, NIHSS and PROMIS-10, for the study will be trained and qualified by rater certification.
Trial data will be captured in a standardised format. The electronic data capturing (EDC) system to be used in the study will be compliant with the Code of Federal Regulation Title 21 part 11.
Prior to the study, the sponsor will ensure that the investigator and institution will permit trial-related monitoring, audits, IRB/EC review and regulatory inspections by providing direct access to source data and study documents. Clinical monitors will ensure that the study is conducted and documented properly according to the protocol, GCP and all applicable regulatory requirements. Monitoring visits and contacts will be made at appropriate times during the study. Clinical monitors must have direct access to source documentation in order to check the completeness, clarity and consistency of the data recorded in the EDC for each patient. The investigator will make available to the clinical monitor all source documents and medical records necessary to review data entered in the EDC and adherence to the protocol, applicable regulations and GCP guidelines.
Discussion
MLC1501 is a third-generation product undergoing investigation in this study. It is a derivative of MLC601 and MLC901 with a combination of only four herbal components, that is, Radix Astragali, Radix Angelicae sinensis, Rhizoma Chuanxiong and Radix Polygalae, that demonstrate similar therapeutic properties of activating molecular mechanisms of protection and repair in neuronal tissue following an ischaemic injury as its predecessors in non-clinical models of cerebral ischaemia.
The MLC1501 study Assessing Efficacy in post-STrOke Subjects with mOtor deficits (MAESTOSO) study of MLC1501 was designed based on recent insights on stroke recovery trials as well as the extensive knowledge gained from earlier studies on MLC601 and MLC901 on the role of baseline stroke severity and treatment window.8–23 The target study population in MAESTOSO are patients with ischaemic stroke who still have clinically important deficits in the postacute period.
In the CHIMES and CHIMES-E studies, patients were included if the NIHSS is between 6 and 14.10 15 The study attempted to exclude very mild cases who would spontaneously recover regardless of treatment and patients who were so severe that they were unlikely to reach functional independence in spite of treatment. The mean NIHSS at baseline of recruited patients was 8.7. Although functional outcome was in favour of MLC601 at 3 months, this did not reach statistical significance most likely due to a ceiling effect. Nearly half of the patients in the placebo group achieved independence (mRS 0–1), more than two-thirds achieved an mRS of 0–2, whereas <5% were deceased or completely disabled at 3 months. This was likely due to exclusion of more severe strokes in the study and may also reflect a general improvement in acute stroke care since the study was designed. Subgroup analysis of treatment effects among patients with NIHSS≥10 showed an adjusted OR of 1.71 (95% CI 1.01 to 2.90, p=0.047) in favour of MLC601 for mRS 0–1 at 3 months.14 Analysis of long-term data from CHIMES-E similarly showed the same increase in treatment effect among patients with NIHSS of ≥10 at 6, 12, 18 and 24 months and particularly when treatment is combined with early rehabilitation.16 18 Further post hoc analysis of patients with baseline NIHSS of 8–14 showed that a sufficient level of motor impairment at study entry, measured by total limb motor score of at least 3 on NIHSS, resulted in a more pronounced and longer lasting treatment effect that reached statistical significance at months 3–24.19 Moreover, previous studies of MLC601 have demonstrated favourable treatment effect on motor recovery as measured by FMA.9 20
In the earliest two clinical trials of MLC601 in China, study patients were recruited from 2 weeks to 6 months of ischaemic stroke, among whom 65% were enrolled within 2 months of stroke.8 The long window of initiation of treatment after a stroke suggests that MLC601 potentially acts mainly on neurorestoration (recovery) rather than neuroprotection (limiting tissue damage) after brain injury. Subsequent studies progressively reduced the recruitment window to 1 month and within 1 week from ischaemic stroke.9 20–22 In the CHIMES and CHIMES-E studies, patients were included within 72 hours of stroke onset.10 15 The mean stroke onset to first dose of study drug was 48 hours. Although functional outcome was in favour of MLC601 at 3 months, this did not reach statistical significance. Subgroup analysis indicated that MLC601 was more likely to benefit patients who were treated beyond 48 hours from stroke onset. Among patients who received study treatment after 48 hours from stroke onset, an adjusted OR of 1.47 (95% CI 1.02 to 2.11, p=0.037) was shown in favour of MLC601 at 3 months.14 Once again, analysis of the long-term data from CHIMES-E showed the same increase in treatment effect among patients with onset to treatment time of >48 hours at 6, 12, 18 and 24 months.16
Based on these analyses, MAESTOSO study eligibility was set to include subjects with NIHSS of 8–18 with concomitant motor deficits who are more than 2 days from ischaemic stroke and are candidates for rehabilitation. Nonetheless, this study design does not imply that patients with milder stroke severity are unlikely to benefit from a potential stroke recovery treatment like MLC1501. Since stroke severity is a consequence of the size of cerebral tissue damage, it would be difficult to imagine that a treatment strategy that improves a certain degree of tissue injury would not benefit smaller or larger lesions. Moreover, we are not advocating that patients who arrive earlier than 48 hours should be delayed from receiving treatment. Rather, patients who arrive in the hospital in the hyperacute and acute periods of stroke should receive standard treatments, such as revascularisation therapies, that are proven to reduce disabilities from stroke. A significant proportion of patients who had a stroke, however, do not arrive within the short window period for such treatment. In addition, deficits remain in many patients who had a stroke in spite of standard treatment. Patients who still exhibit neurological deficits in the postacute period are less likely to achieve complete long-term recovery and would benefit most from neurorestorative therapy. The proposed postacute time window for MLC1501 is meant to identify these patients who have persisting deficits resulting from the stroke.
Stroke is a major cause of death and disability with only a limited number of treatment options.28 Many patients do not receive time-sensitive acute stroke therapies for various reasons.29 30 There is a need for interventions that can enhance recovery beyond the acute period of stroke. Proving clinical efficacy and safety of MLC1501 on long-term recovery in patients suffering from ischaemic stroke in a well-designed pragmatic clinical trial will clarify its role as a recovery treatment.
Conclusions
MLC1501 has a good safety profile as well as potential to be a treatment for recovery after brain injury. The MAESTOSO Study is designed as a recovery trial in patients who have suffered an ischaemic stroke. The results of this study will provide objective level B evidence on the efficacy of MLC1501 on long-term recovery and safety of 24 weeks of treatment among patients with ischaemic stroke.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the respective ethics committees or institutional review boards of Baguio General Hospital and Medical Center, Manila Doctors Hospital, Metro Davao Medical Center, Southern Philippines Medical Center, West Visayas State University Medical Center, University of Santo Tomas Hospital, Quirino Memorial Medical Center, Jose R Reyes Memorial Medical Center, National Neuroscience Institute–Singapore General Hospital Campus, National Neuroscience Institute–Tan Tock Seng Campus and Changi General Hospital (study reference ID: EFSA20221_01). Participants gave informed consent to participate in the study before taking part.
Footnotes
Contributors CPLHC, RUE, CGE, JHH, JMA, COCC, MEVC, AYL, Y-HK, DADS, CHT, JKL and NV all contributed equally to the protocol development, study conceptualisation, manuscript writing and approval of submission of the article. CPLHC acted as the guarantor.
Funding This study was funded by Moleac Pte. Ltd., Singapore
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.