Abstract
Background Stroke remains a major global health challenge, with China experiencing a significant burden due to its high incidence and severe outcomes. Reperfusion therapies, such as intravenous thrombolysis and endovascular thrombectomy, have shown substantial benefits in improving early outcomes for ischaemic stroke. Recent clinical trials have validated the safety and efficacy of a broader range of thrombolytic agents and expanded the eligible patient populations for both intravenous thrombolysis and mechanical thrombectomy. This guideline aims to provide the latest evidence-based insights in the field of reperfusion therapy.
Methods The Chinese Stroke Association (CSA) established a writing group to develop updated guidelines on reperfusion therapy for acute ischaemic stroke. A comprehensive search of MEDLINE (via PubMed) was conducted up to 30 September 2024. Experts in the field of stroke engaged in extensive discussions, both online and offline, to evaluate the latest evidence. Each recommendation was graded using the CSA’s class of recommendation and level of evidence in the Guideline Development Manual of the CSA.
Results This guideline, reviewed and approved by the CSA Guidelines Writing Group, outlines the criteria for patient selection for thrombolysis and thrombectomy and summarises the latest evidence on various thrombolytic drug options to support decision-making in reperfusion therapy. Additionally, the guideline includes green channel flow charts for intravenous thrombolysis and mechanical thrombectomy, designed to assist clinicians in optimising their clinical decisions.
Conclusion This guideline updates the latest advancements in the field of reperfusion therapy for acute ischaemic stroke. It is anticipated that future clinical research will further advance areas such as innovative thrombolytic agents, expanded indications for thrombolysis and mechanical thrombectomy.
Introduction
Stroke is one of the leading causes of death worldwide and in China, characterised by high mortality and high disability rates.1 In China, approximately 2 million new cases of stroke occur annually, with ischaemic stroke accounting for about 80% of these cases,2 imposing a significant burden on families and society. In the early phase of ischaemic stroke, reperfusion therapies, including intravenous thrombolysis and endovascular thrombectomy, can restore cerebral blood flow and significantly improve functional outcomes at 3 months.3 4
Recently, significant progress has been made in clinical trials within the fields of thrombolysis and thrombectomy. The latest trial results have continuously expanded the eligible patient population for both thrombolysis and thrombectomy. Based on these recent research findings, the Chinese Stroke Association (CSA) established a writing group to perform a comprehensive search of MEDLINE (via PubMed) as of 30 September 2024. Experts in the field of stroke were invited to extensively discuss. Each evidence was graded and recommended according to the CSA class of recommendation and level of evidence in the Guideline Development Manual of the CSA (figure 1), and the CSA Guideline on reperfusion therapy for acute ischaemic stroke was finally formed. This guideline outlines the criteria for selecting treatment populations for thrombolysis and thrombectomy and presents the current evidence regarding various thrombolytic drug options. We also designed a green channel flow chart for intravenous thrombolysis (figure 2) and mechanical thrombectomy (figure 3) in patients with acute ischaemic stroke. We hope we can help neurologists, neurosurgeons and emergency department physicians make decisions about reperfusion therapy.
CSA class of recommendation and level of evidence to clinical strategies in patient with acute ischaemic stroke. CSA, Chinese Stroke Association. RCT, randomised controlled trial.
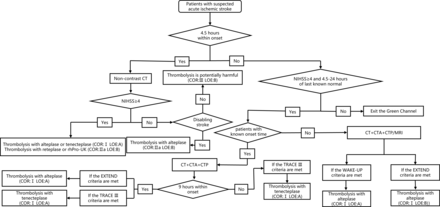
Green channel flow chart for intravenous thrombolysis. Disabling stroke was defined as complete hemianopsia (≥2 on the NIHSS Question #3), or severe aphasia (≥2 on NIHSS Question #9), or visual or sensory extinction (≥1 on NIHSS Question #11), or any weakness limiting sustained effort against gravity (≥2 on NIHSS Question #6 or #7) or any consciousness disorder (≥1 on NIHSS Question #1a) EXTEND imaging criteria: (1) Infarct core volume < 70 mL. (2) Hypoperfused volume/infarct core volume (Tmax >6 s on CTP or MRI perfusion) ≥1.2. (3) Mismatch volume ≥10 mL. WAKE-UP imaging criteria: (1) An ischaemic lesion that was visible on MRI DWI but no parenchymal hyperintensity on FLAIR (DWI-FLAIR mismatch). TRACE 3 imaging criteria: (1) Large vessel occlusion of internal carotid artery or M1 or M2 segments of middle cerebral artery. (2) Infarct core volume<70 mL. (3) Hypoperfused volume/Infarct core volume (Tmax >6 s on CTP or MRI perfusion) ≥1.8. (4) Mismatch volume ≥15 mL. COR, class of recommendation; CTA, CT angiography; CTP, CT perfusion; DWI, diffusion weighted imaging; EXTEND, Extending the Time for Thrombolysis in Emergency Neurological Deficits; FLAIR, fluid attenuated inversion recovery; LOE, level of evidence; NIHSS, National Institute of Health Stroke Scale; rhPro-UK, recombinant human prourokinase; TRACE-Ⅲ, Teneteplase Reperfusion Therapy in Acute Ischaemic Cerebrovascular Events-III; WAKE-UP, Efficacy and Safety of MRI-based Thrombolysis in Wake-up Stroke.
Green channel flow chart for mechanical thrombectomy. ASPECTS, Alberta Stroke Programme Early CT Score; COR, class of recommendation; CTA, CT angiography; LOE, level of evidence; NIHSS, National Institute of Health Stroke Scale; pc-ASPECTS, posterior circulation Acute Stroke Prognosis Early CT Score; rhPro-UK, recombinant human prourokinase.
Thrombolysis
Thrombolysis within 4.5 hours of onset
For patients with ischaemic stroke within 4.5 hours of onset, does intravenous thrombolysis lead to better functional outcomes than control group? Which thrombolytic agents are available for intravenous thrombolytic therapy in these patients?
Analysis of current evidence
An updated Cochrane systematic review and an individual patient meta-analysis by the Stroke Thrombolysis Trialists’ Collaboration5–7 showed that among patients with acute ischaemic stroke within 4.5 hours after symptom onset, and with National Institute of Health Stroke Scale (NIHSS)≥6, treatment with intravenous alteplase guided by non-contrast CT (NCCT) significantly increased the rate of a favourable functional outcome (modified Rankin Scale, mRS 0–2) at 3 months (OR 1.17, 95% CI 1.06 to 1.29; p<0.01).
Tenecteplase is a single bolus thrombolytic agent with higher fibrin specificity and a longer half-life than alteplase. 12 randomised controlled trials (RCTs) have compared tenecteplase with alteplase in people with acute ischaemic stroke identified in MEDLINE.8–19 No single trial has demonstrated that tenecteplase leads to greater recovery than alteplase in unselected patients. A 2019 meta-analysis20 concluded that tenecteplase was non-inferior to alteplase (mRS 0–1 at 3 months: risk difference (RD) 4%, 95% CI −1% to 8%) but this was confounded by the significant contribution of the Study of Tenecteplase versus Alteplase for Thrombolysis in Acute Ischaemic Stroke (NOR-TEST) trial which used a higher dose of 0.4 mg/kg and included a substantial proportion (18%) of people with stroke mimics.12 The NOR-TEST trial did not show a better prognosis with tenecteplase than with alteplase (OR 1.08, 95% CI 0.84 to 1.38, p=0.52), but the two showed similar safety outcomes (the frequency of serious adverse events, 26% vs 26%, p=0.74). A subsequent trial of 0.4 mg/kg tenecteplase in patients with moderate-severe ischaemic stroke showed this higher dose led to higher rates of intracerebral haemorrhage than alteplase (NOR-TEST 2, part A) (unadjusted OR 3.68, 95% CI 1.49 to 9.11, p<0.01),11 and this dose is no longer recommended. Tenecteplase (0.25 mg/kg) delivered in mobile stroke units (MSU) setting (Tenecteplase vs Alteplase for Stroke Thrombolysis Evaluation Trial in the Ambulance, TASTE-A)8 led to better measures of imaging reperfusion than alteplase (adjusted incidence rate ratio 0.55, 95% CI 0.37 to 0.81, p<0.01) but the study was inadequately powered to test any difference in outcomes (mRS 5–6 at 3 months: adjusted OR (aOR) 0.70, 95% CI 0.23 to 2.16, p=0.54). Alteplase Compared with Tenecteplase in Patients With Acute Ischaemic Stroke (AcT) (mRS 0–1 at 3–4 months: unadjusted RD 2.1%, 95% CI 2.6% to 6.9%) and Tenecteplase Reperfusion Therapy in Acute Ischaemic Cerebrovascular Events-II (TRACE-II) (mRS 0–1 at 3 months: relative risk (RR) 1.07, 95% CI 0.98 to 1.16) trial demonstrated that tenecteplase (0.25 mg/kg) was non-inferior to alteplase (0.9 mg/kg) for excellent functional outcomes (mRS 0–1) at 3 months with a similar safety profile.14 18 The findings in TASTE trial provide further evidence to strengthen the assertion of the non-inferiority of tenecteplase to alteplase, specifically when perfusion imaging has been used to identify reperfusion-eligible patients with stroke (mRS 0–1 at 3 months: standardised RD 0.03, 95% CI 0.03 to 0.10).17
Therefore, five large randomised trials (AcT, TRACE-2, TASTE, alteplase-tenecteplase trial evaluation for stroke thrombolysis[ATTEST-2], and a study in Chinese patients to compare how tenecteplase and alteplase given after a stroke improve recovering of physical activity[ORIGINAL]) have demonstrated that tenecteplase 0.25 mg/kg is non-inferior to alteplase for excellent clinical outcomes when delivered within 4.5 hours of stroke onset.
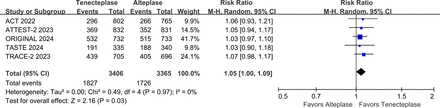
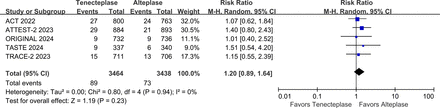
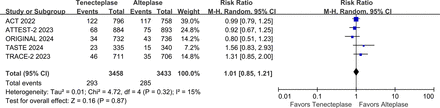
The writing group conducted a meta-analysis that included five large phase III RCTs (AcT, TRACE-2, TASTE, ATTEST 2, ORIGINAL) that compared the efficacy and safety of tenecteplase vs alteplase within 4.5 hours of stroke onset.10 13 14 17 18 The results showed that the proportion of patients with mRS 0–1 at 3 months was higher (RR 1.05, 95% CI 1.01 to 1.09, I2=0%) in the tenecteplase group than in the alteplase group (figure 4), and there was no significant difference in symptomatic intracranial haemorrhage (sICH) (RR 1.20, 95% CI 0.89 to 1.64, I2=0%) (figure 5) and 3-month mortality (RR 1.01, 95% CI 0.85 to 1.21, I2=15%) between the two groups (figure 6).
Forest plot of excellent functional outcome (mRS 0-1) at 3 months in patients with acute ischaemic stroke who received tenecteplase within the 4.5 hours time window, compared with the control group. AcT, Alteplase Compared with Tenecteplase in Patients With Acute Ischaemic Stroke; ATTEST-2, alteplase-tenecteplase trial evaluation for stroke thrombolysis; mRS, modified Rankin Scale; ORIGINAL, a study in Chinese patients to compare how tenecteplase and alteplase given after a stroke improve recovering of physical activity; TASTE, Tenecteplase versus Alteplase for Stroke Thrombolysis Evaluation; TRACE-II, tenecteplase Reperfusion Therapy in Acute Ischaemic Cerebrovascular Events-II.
Forest plot of symptomatic intracranial haemorrhage rates in patients with acute ischaemic stroke who received tenecteplase within the 4.5 hours time window, compared to the control group. AcT, Alteplase Compared with Tenecteplase in Patients With Acute Ischaemic Stroke; ATTEST-2, alteplase-tenecteplase trial evaluation for stroke thrombolysis; ORIGINAL, a study in Chinese patients to compare how tenecteplase and alteplase given after a stroke improve recovering of physical activity. TASTE, Tenecteplase versus Alteplase for Stroke Thrombolysis Evaluation; TRACE-II, tenecteplase Reperfusion Therapy in Acute Ischaemic Cerebrovascular Events-II.
Forest plot of mortality at 3 months in patients with acute ischaemic stroke who received tenecteplase within the 4.5 hours time window, compared to the control group. AcT, Alteplase Compared with Tenecteplase in Patients With Acute Ischaemic Stroke; ATTEST-2, alteplase-tenecteplase trial evaluation for stroke thrombolysis; ORIGINAL, a study in Chinese patients to compare how tenecteplase and alteplase given after a stroke improve recovering of physical activity. TASTE, Tenecteplase versus Alteplase for Stroke Thrombolysis Evaluation; TRACE-II, tenecteplase Reperfusion Therapy in Acute Ischaemic Cerebrovascular Events-II.
Reteplase is a recombinant plasminogen activator that is characterised by a double-bolus approach (the boluses are separated by 30 min) with a fixed dose regimen.21–23 In a phase 2, RCT, the proportion of patients with an excellent functional outcome was higher with two 18 mg doses of reteplase than with two 12 mg doses or with alteplase at a dose of 0.9 mg per kilogram of body weight, and the higher dose of reteplase was not associated with an increased risk of fatal bleeding.24 In the Reteplase treating patients with acute ischemic stroke (RAISE) trial involving patients with acute ischaemic stroke who were eligible for intravenous thrombolysis within 4.5 hours after they were last known to be well, reteplase was superior to alteplase with respect to an excellent functional outcome at 3 months (mRS 0–1 at 3 months, RR 1.13, 95% CI 1.05 to 1.21, p<0.01). However, patients receiving reteplase had a higher incidence of any intracranial haemorrhage than those receiving alteplase (7.7% vs 4.9%, RR 1.59, 95% CI 1.00 to 2.51)25 Therefore, for patients with an acute ischaemic stroke within the 4.5 hours onset window, intravenous thrombolysis with reteplase has been proven superior to alteplase.
Recombinant human prourokinase (rhPro-UK)26 is a genetically engineered drug, which is a non-tissue plasminogen activator. Its structure is a single peptide chain, and it has a long half-life and can play a continuous role in thrombolysis. Recombinant human prourokinase in Acute Ischaemic Stroke Within 4.5 Hours of Stroke Onset Trial 2, a phase III trial comparing the safety and efficacy of rhPro-UK 35 mg versus alteplase 0.9 mg/kg in patients with acute ischaemic stroke within 4.5 hours of onset, enrolled 1552 subjects. rhPro-UK was non-inferior to alteplase (RR 1.04, 95% CI 0.98 to 1.10, p=0.24) in excellent functional outcome (mRS 0–1) at 3 months, and had a lower rate of sICH within 36 hours (RD −1.0, 95% CI −2.1 to −0.1, p=0.02). The 3-month mortality did not have a significantly difference between the two groups (RD −0.8, 95% CI −2.6 to 1.1, p=0.41).
Recommendation
For patients with acute ischaemic stroke and National Institute of Health Stroke Scale (NIHSS)≥4, in whom treatment can be started within 4.5 hours of known onset, thrombolysis with alteplase or tenecteplase should be performed.
Level of evidence: A
Class of recommendation: I
Recommendation:
For patients with acute ischaemic stroke and NIHSS≥4, in whom treatment can be started within 4.5 hours of known onset, thrombolysis with reteplase or recombinant human prourokinase can be beneficial.
Level of evidence: B
Class of recommendation: Ⅱa
Thrombolysis between 4.5 and 24 hours of onset
Mismatch in perfusion imaging can quantify penumbral tissue and identify patients who might benefit from intravenous alteplase beyond 4.5 hours. However, most RCTs of intravenous thrombolysis in extended time windows allowed both patients with known onset times >4.5 hours and patients with wake-up stroke (whose true onset time may frequently be <4.5 hours before randomisation) or unknown onset stroke. We decided to provide separate recommendations for these two different clinical scenarios.
In patients with ischaemic stroke of known onset time between 4.5 and 9 hours or with an unknown onset, does intravenous thrombolysis result in better functional outcomes compared with control group?
Analysis of current evidence
The Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET),27 a phase II study, prospectively and randomly assigned 101 patients with NIHSS>4 to receive alteplase (n=52) or placebo (n=49) 3–6 hours after onset of ischaemic stroke. The results indicated reperfusion occurred more frequently with alteplase than with placebo (RD 30%, 95% CI 9% to 51%, p=0.01). However, no significant difference was observed between the two groups in achieving favourable functional outcome (mRS 0–2: RD 5%, 95% CI –15% to 27%, p=0.66).
The European Cooperative Acute Stroke Study 4 (ECASS-4) studied whether patients with acute ischaemic stroke with NIHSS 4–26 and penumbral tissue identified on MRI 4.5–9 hours after symptom onset benefit from alteplase compared with placebo.28 The trial was stopped early for slow recruitment. Of the 119 (61 in the alteplase group and 58 in the placebo group) enrolled patients, 37 (31%) had known stroke duration of 4.5–9 hours (with a median time to treatment of 6.9 hours) and 82 (69%) were wake-up strokes. The primary endpoint showed no significant difference in the distribution of mRS at 3 months (OR 1.20, 95% CI 0.63 to 2.27, p=0.58).
The Extending the Time for Thrombolysis in Emergency Neurological Deficits (EXTEND) trial compared alteplase with placebo in patients presenting between 4.5 and 9 hours after stroke onset or on awakening with stroke (if within 9 hours from the midpoint of sleep).29 Patients with NIHSS 4–26 were selected based on CT perfusion (CTP) or MRI perfusion weighted imaging/diffusion weighted imaging (MRI PWI/DWI) core/perfusion mismatch criteria, including an ischaemic core volume (relative cerebral blood flow <30% on CTP or Apparent Diffusion Coefficient<620 mm2/s on diffusion MRI) ≤70 mL, an absolute difference between the volume of hypoperfusion and the volume of the ischaemic core ≥10 mL and a perfusion-to-core mismatch ratio ≥1.2. Of the 225 enrolled patients (113 alteplase, 112 placebo), 146 (65%) had awoken with stroke symptoms. The study showed that alteplase was significantly associated with a higher rate of achieving the primary outcome (mRS score of 0–1) at 3 months (adjusted RR 1.44, 95% CI 1.01 to 2.06, p=0.04), whereas the 90-day mortality rate showed no significant difference between the two groups (adjusted RR 1.17, 95% CI 0.57 to 2.40, p=0.67).
A meta-analysis of individual participant data from the EPITHET, ECASS-4 and EXTEND trials included 414 patients who met the inclusion criteria (52% imaged with MRI PWI/DWI and 48% with CTP).30 The analysis found that in patients with ischaemic stroke 4.5–9 hours after onset or wakeup stroke who were imaged with CTP or PWI, intravenous alteplase was significantly associated with a higher rate of excellent outcomes (mRS score of 0–1) (adjusted OR 1.86, 95% CI 1.15 to 2.99, p=0.01). Notably, 61% of patients in this analysis would be eligible for thrombectomy on the basis of the presence of a proximal large vessel occlusion (LVO). Therefore, the evidence-based recommendation only applies to patients who will not undergo mechanical thrombectomy (MT).
Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke (WAKE-UP) trial randomly assigned patients who had an unknown time of onset of stroke to receive either intravenous alteplase 0.9 mg/kg (n=254) or matching placebo (n=249).31 The time that had elapsed since the patient was last known to be well had to be more than 4.5 hours (with no upper limit). All the patients had an ischaemic lesion that was visible on DWI but no parenchymal hyperintensity on Fluid Attenuated Inversion Recovery (FLAIR), indicating a DWI-FLAIR mismatch, which in turn suggested that the stroke had occurred approximately within the previous 4.5 hours. The trial excluded patients who were scheduled to undergo thrombectomy. Alteplase was associated with a higher percentage of favourable outcomes (mRS score of 0–1) at 3 months (adjusted OR 1.61, 95% CI 1.09 to 2.36, p=0.02).
The Thrombolysis for Acute Wake-Up and Unclear-Onset Strokes With Alteplase at 0.6 mg/kg (THAWS) trial was an investigator-initiated, multicentre, randomised, open-label, blinded-end point trial. The trial used the same criteria for patient selection as the WAKE-UP trial, and patients with DWI-FLAIR mismatch were randomly assigned (1:1) to receive the low-dose alteplase (0.6 mg/kg) or control group.32 The results found no significant difference in favourable outcomes (mRS score of 0–1) between the alteplase group and the control group (RR 0.97, 95% CI 0.68 to 1.41, p=0.89).
A systematic review and meta-analysis based on the Evaluation of Unknown Onset Stroke Thrombolysis (EOS), was conducted for RCTs comparing intravenous alteplase with placebo in patients with stroke with an unknown time of onset.33 The trials included adults with stroke of unknown onset time who were assessed using DWI/PWI, CTP or MRI with DWI-FLAIR mismatch. A total of 843 individuals from four trials met our eligibility criteria for inclusion: WAKE-UP, EXTEND, THAWS and ECASS-4. Compared with placebo, intravenous alteplase was significantly associated with a favourable functional outcome (adjusted OR 1.49, 95% CI 1.10 to 2.03, p=0.01, I²=27%). In summary, intravenous thrombolysis with alteplase is effective for patients with acute ischaemic stroke occurring on awakening or with an unknown onset time when selected based on advanced imaging (CTP or MRI).
The Tenecteplase in Wake-up Ischaemic Stroke Trial (TWIST) randomly assigned 578 patients with wake-up stroke, selected by non-contrast CT alone with no intracranial haemorrhage detected, to receive either a single intravenous bolus of tenecteplase 0.25 mg/kg (n=288) or control (no thrombolysis; n=290) within 4.5 hours of awakening.34 The distribution of mRS scores, the primary outcome, showed no significant difference in the 3-month functional outcome between the tenecteplase group and the control group (adjusted OR 1.18, 95% CI 0.88 to 1.58, p=0.27). Therefore, the use of tenecteplase for intravenous thrombolysis in patients with acute ischaemic stroke occurring on awakening or with an unknown onset time requires further investigation.
Recommendation
For patients with ischaemic stroke of known onset time between 4.5 and 9 hours, and with National Institute of Health Stroke Scale (NIHSS) 4–26, who have a CT or MRI core/perfusion mismatch*, we recommend intravenous thrombolysis with alteplase if MT is neither indicated nor planned.
*The core/perfusion mismatch was assessed with an automated software and defined as follows:
Infarct core volume <70 mL
Hypoperfused volume/infarct core volume ratio ≥1.2
Mismatch volume ≥10 mL
Level of evidence: A
Class of recommendation: I
For patients with wake-up stroke who were last known to be well more than 4.5 hours prior and have an MRI diffusion weighted imaging-fluid attenuated inversion recovery mismatch, we recommend intravenous thrombolysis with alteplase if mechanical thrombectomy is neither indicated nor planned.
Level of evidence: A
Class of recommendation: I
For patients with wake-up stroke who have a CT or MRI core/perfusion mismatch* within 9 hours from the midpoint of sleep, with NIHSS 4–26, and for whom MT is neither indicated nor planned, we recommend intravenous thrombolysis with alteplase.
*The core/perfusion mismatch was assessed with an automated software and defined as follows:
Infarct core volume <70 mL
Hypoperfused volume/infarct core volume ratio ≥1.2
Mismatch volume ≥10 mL
Level of evidence: B
Class of recommendation: I
For patients with wake-up stroke or ischaemic stroke of unknown onset time, selected with no brain imaging other than plain CT, we recommend against intravenous thrombolysis with tenecteplase 0.25 mg/kg except clinical trial.
Level of evidence: B
Class of recommendation: Ⅲ
In patients with moderate-to-severe stroke with large vessel occlusion of 4.5–24 hours duration, does intravenous thrombolysis with tenecteplase lead to better functional outcome?
Analysis of current evidence
Data are lacking on the safety and efficacy of thrombolytics in patients with core/perfusion mismatch up to 24 hours. The phase III, placebo-controlled Tenecteplase in Patients with Stroke Between 4.5 and 24 Hours (TIMELESS) trial included 458 patients with acute stroke within an extended time window of 4.5–24 hours treated with 0.25 mg/kg tenecteplase (n=228) or placebo (n=230).35 The neuroimaging inclusion criteria were internal carotid artery (ICA), M1 or M2 segment of the middle cerebral artery (MCA) occlusion identified by magnetic resonance angiography/CT angiography (CTA) with target mismatch profile on CTP or PWI (infarct core volume <70 mL, mismatch ratio ≥1.8, and mismatch volume ≥15 mL). In the primary efficacy end-point analysis, there was no significant difference in the odds of a lower mRS score at 3 months (common OR 1.13, 95% CI 0.82 to 1.57, p=0.45).
Tenecteplase Reperfusion Therapy in Acute Ischaemic Cerebrovascular Events–III (TRACE-III) trial investigated the efficacy and safety of tenecteplase administered 4.5–24 hours after stroke onset in patients who did not have access to MT.36 The trial involved 516 Chinese patients with occlusion of the ICA or the M1 or M2 segment of the MCA who had a score of 6–25 on the NIHSS score and had evidence of salvageable tissue on perfusion imaging assessed with the iStroke software (defined as in the TIMELESS study). Patients were randomly assigned to receive 0.25 mg/kg tenecteplase (n=264) or placebo (n=252). Tenecteplase resulted in a higher percentage of patients with good outcomes (mRS score of 0–1) than placebo at 3 months (RR 1.37, 95% CI 1.04 to 1.81, p=0.03). The TRACE-III trial, compared with the TIMELESS trial, excluded patients who were planned for MT. Given that a large proportion of patients worldwide (including in developed countries) cannot reach hospitals with thrombectomy capabilities within the effective treatment window, the TRACE-III trial also demonstrates better applicability compared with the TIMELESS trial.
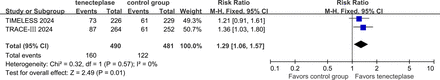
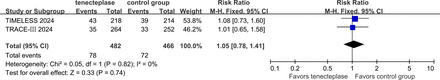
The writing group further conducted a meta-analysis of study-level data from the two trials and found a statistically significant difference in the proportion of patients with a good outcome (mRS score of 0–1) at 3 months in favour of tenecteplase (RR 1.29, 95% CI 1.06 to 1.57, I2=0%) (figure 7), indicating that tenecteplase is superior to the control group for this endpoint. Additionally, there were no significant differences between the two groups in terms of sICH rates (RR 2.08, 95% CI 0.85 to 5.06, I2=10%) (figure 8) or 3-month mortality (RR 1.05, 95% CI 0.78 to 1.41, I2=0%), indicating similar safety outcomes (figure 9).
Forest plot of excellent functional outcome (mRS 0-1) at 3 months in patients with acute ischaemic stroke with anterior circulation LVO who received tenecteplase within the 4.5–24 hours time window, compared to the control group. LVO, large vessel occlusion; mRS, modified Rankin Scale; RR, risk ratio; TIMELESS; Tenecteplase in Patients with Stroke Between 4.5 and 24 Hours; TRACE-III, Tenecteplase Reperfusion Therapy in Acute Ischaemic Cerebrovascular Events–III.
Forest plot of symptomatic intracranial haemorrhage rates within 72 hours in patients with anterior circulation LVO who received tenecteplase within the 4.5–24 hours time window, compared to the control group. LVO, large vessel occlusion; RR, risk ratio; TIMELESS; Tenecteplase in Patients with Stroke Between 4.5 and 24 Hours; TRACE-III, Tenecteplase Reperfusion Therapy in Acute Ischaemic Cerebrovascular Events–III.
Forest plot of mortality at 3 months in patients with anterior circulation LVO who received tenecteplase within the 4.5–24 hours time window, compared with the control group. LVO, large vessel occlusion; RR, risk ratio; TIMELESS; Tenecteplase in Patients with Stroke Between 4.5 and 24 Hours; TRACE-III, Tenecteplase Reperfusion Therapy in Acute Ischaemic Cerebrovascular Events–III.
Recommendation
For patients with National Institute of Health Stroke Scale (NIHSS) score ≥6 and an onset time between 4.5 and 24 hours, with large vessel occlusion in the anterior circulation, and perfusion imaging indicating a core/perfusion mismatch*, but who cannot undergo mechanical thrombectomy, we recommend intravenous thrombolysis with 0.25 mg/kg tenecteplase.
*The core/perfusion mismatch was assessed with an automated software and defined as follows:
Infarct core volume <70 mL
Hypoperfused volume/infarct core volume ratio ≥1.8
Mismatch volume ≥15 mL
Level of evidence: A
Class of recommendation: I
Additional information
The CHinese Acute Tissue-Based Imaging Selection for Lysis In Stroke-Tenecteplase II trial (CHABLIS-T II, NCT04516993)37 has revealed results in the International Stroke Conference 2024 and suggested that for patients with large vessel occlusion presenting within 4.5–24 hours, 0.25 mg/kg tenecteplase significantly improved reperfusion compared with the control group (adjusted risk ratio (aRR) 3.0, 95% CI 1.5 to 5.7, p<0.01). The ongoing Extending the Time Window for Tenecteplase by Effective Reperfusion in Patients With Large Vessel Occlusion trial (ETERNAL-LVO, NCT04454788) conducted in Australia will explore the efficacy and safety of 0.25 mg/kg tenecteplase compared with no intravenous thrombolytic treatment or intravenous 0.9 mg/kg alteplase in patients with acute large vessel occlusion stroke up to 24 hours.
In the extended time window of 4.5–24 hours duration, the Randomisation to Extend Stroke Intravenous ThromboLysis In Evolving Non-Large Vessel Occlusion With TNK trial (RESILIENT, NCT05199662) will investigate the efficacy of tenecteplase treatment in non-large vessel occlusion stroke. Meanwhile, the Extending the Time Window for Tenecteplase by Recanalisation of Basilar Artery Occlusion in Posterior Circulation Stroke trial (POST-ETERNAL, NCT05105633) and the Tenecteplase Reperfusion Therapy in Acute Ischaemic Cerebrovascular Events-5 trial (TRACE-5, NCT06196320) were oriented on the basilar artery occlusion in posterior circulation stroke. These ongoing trials are expected to provide more evidence for thrombolytic therapy in specific stroke populations.
Mechanical thrombectomy
In patients with ischaemic stroke due to LVO of known onset time within 24 hours, does MT lead to better functional outcomes compared with best medical management (BMM)?
Currently, Extending the Time for Thrombolysis in Emergency Neurological Deficits—Intra-Arterial (EXTEND IA), Endovascular Treatment for Small Core and Proximal Occlusion Ischaemic Stroke (ESCAPE), Endovascular Revascularisation With Solitaire Device versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours (REVASCAT), Solitaire With the Intention For Thrombectomy as PRIMary Endovascular Treatment Trial (SWIFT PRIME), Endovascular treatment for acute ischaemic stroke in the Netherlands (MR CLEAN) these five RCTs demonstrated that MT was superior to BMM for proximal LVO in the anterior circulation including intracranial ICA and MCA occlusions within 6 hours after onset, the favourable functional outcome of MT at 3 months (mRS 0–2) is better than that of BMM.38–42 Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) study which was the pooled analysis of individual patient data level from the five RCTs also found the same findings (MT vs BMM, 90 days mRS: adjusted cOR 2.49, 95% CI 1.76 to 3.53; p<0.01).3
Endovascular Therapy Following Imaging Evaluation for Ischaemic Stroke (DEFUSE-3)43 found that MT for ischaemic stroke 6–16 hours after patients were last known to be well resulted in better functional outcomes at 3 months (mRS 0–2) than BMM among patients with proximal MCA or ICA occlusion and a region of tissue that was ischaemic but not yet infarcted (90 days mRS: cOR 2.77, 95% CI 1.63 to 4.70, p<0.01). The imaging criteria were initial infarct volume (ischaemic core) of less than 70 mL, a ratio of volume of ischaemic tissue to initial infarct volume of 1.8 or more and an absolute volume of potentially reversible ischaemia (penumbra) of 15 mL or more.
Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN)44 found that among patients with acute stroke who had last been known to be well 6–24 hours earlier and who had a mismatch between clinical deficit and infarct, the favourable functional outcome at 3 months (mRS 0–2) was better with MT than with BMM (adjusted difference 33. 95% CI 21 to 44). The enrolled criteria were: Group A were 80 years of age or older, had a score of 10 or higher on the NIHSS scores and had an infarct volume of less than 21 mL; Group B were younger than 80 years of age, had a score of 10 or higher on the NIHSS and had an infarct volume of less than 31 mL; Group C were younger than 80 years of age, had a score of 20 or higher on the NIHSS and had an infarct volume of 31 to less than 51 mL.
Thrombectomy for anterior circulation stroke beyond 6 hours from time last known well (AURORA) study confirmed that the proportion of mRS 0–2 at 90 days in patients who met the criteria of DEFUSE-3 or DAWN study was significantly improved by MT (adjusted common odds ratio (cOR) 2.54, 95% CI 1.83 to 3.54, p<0.01).45
Endovascular treatment of acute ischaemic stroke in the Netherlands for late arrivals (MR CLEAN LATE)46 found that MT was effective and safe for patients with ischaemic stroke caused by an anterior circulation LVO who presented 6–24 hours from onset or last seen well (90 days mRS, cOR 1.67, 95% CI 1.20 to 2.32). The study assessed collateral blood flow in the affected MCA territory using CTA, either single-phase or arterial phase of multiphase CTA, comparing it with the contralateral side. Only patients with collateral blood flow were included. The collateral flow was categorised into three grades: Grade 1, with collateral filling ≤50% but >0%; Grade 2, with collateral filling >50% but <100%; and Grade 3, with collateral filling at 100%.
Six RCTs have demonstrated that MT is superior to BMM in anterior circulation LVO patients with large infarcts.47–51 However, the imaging modes of baseline large infarcts were different among the six trials.
Randomised Controlled Trial of Endovascular Therapy for Acute Large Vessel Occlusion With Large Ischaemic Core (RESCUE-Japan LIMIT)51 is the first RCT to demonstrate MT is superior to BMM for the functional independence outcome (mRS 0–3) at 3 months (RR 2.43, 95% CI 1.35 to 4.37, p<0.01), which included patients who had ICA or M1 occlusions with Alberta Stroke Programme Early Computed Tomographic Score (ASPECTS) score 3–5 by DWI or NCCT within 6 hours of time from onset to randomisation, or patients without signal changes on the initial FLAIR images and ASPECTS score 3–5 within 6–24 hours of time from onset to randomisation.
The Study of Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core (ANGEL-ASPECT)49 trial is another RCT to find large infarct patients within 24 hours of time from onset to randomisation who had ASPECTS score 3–5 on NCCT or ASPECTS score 0–2 on NCCT with infarct-core volume 70–100 mL. This trial found that patients with large infarcts had better favourable functional outcome at 3 months (mRS 0–2) with MT than with BMM (a shift in the distribution of mRS scores at 3 months, OR 1.37, 95% CI 1.11 to 1.69, p=0.01).
A Randomised Controlled Trial to Optimise Patient’s Selection for Endovascular Treatment in Acute Ischaemic Stroke (SELECT 2)50 trial found that large infarct patients with ASPECTS score 3–5 on NCCT or infarct-core volume >50 mL within 24 hours could benefit from MT, the probability of achieving favourable functional outcome (mRS 0–2) at 3 months increased by approximately three times (RR 2.97, 95% CI 1.60 to 5.51).
The Efficacy and Safety of Thrombectomy in Stroke With Extended Lesion and Extended Time Window (TENSION)47 trial found that large infarct patients with ASPECTS score 3–5 on NCCT or DWI within 12 hours could significantly improve favourable functional outcome (mRS 0–2) at 3 months through MT (adjusted OR 7.16, 95% CI 2.12 to 24.21).
Large Stroke Therapy Evaluation (LASTE)48 trial found that the disability rate at 3 months (mRS≥4) in the MT group is significantly lower in large infarct patients with ASPECTS score 0–5 on NCCT or DWI within 6.5 hours could benefit from MT (generalised OR 1.63, 95% CI 1.29 to 2.06, p<0.01).
Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischaemic Stroke (TESLA)52 trial used NCCT to screen for patients with a large infarct core and ASPECTS scores of 2–5. The study found that MT leads to better functional outcome at 3 months (mRS 0–2) compared with BMM (RR 1.64, 95% CI 0.91 to 2.16, p=0.09). The meta-analysis of these six RCTs53 also found MT is superior to BMM for large ischaemic infarct (mRS 0–3 at 3 months: RR 1.78, 95% CI 1.28 to 2.48).
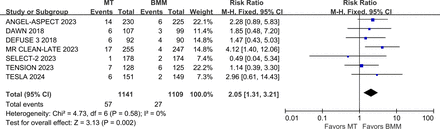
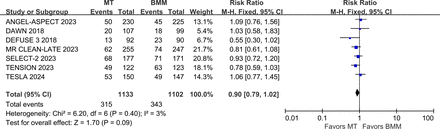
The writing group conducted a meta-analysis of clinical trials (including DEFUSE-3, DAWN, MR CLEAN LATE, SELECT-2, ANGEL-ASPECT, TENSION and TESLA)43 44 46 47 49 50 52 involving patients with ischaemic stroke due to LVO who underwent MT beyond the conventional time window. Results indicated that the proportion of favourable functional outcome (mRS 0–2) was significantly higher in the MT group compared with the control group (RR 2.46, 95% CI 1.56 to 3.88, I2=83%)(figure 10). However, the rate of sICH was also significantly higher in the MT group (RR 2.05, 95% CI 1.31 to 3.21, I2=0%) (figure 11), while 3-month mortality rates showed no significant difference between the groups (RR 0.90, 95% CI 0.79 to 1.02, I2=3%) (figure 12). These findings indicate that MT is effective within the 6–24 hours window after symptom onset.
Forest plot of favourable functional outcome (mRS 0–2) at 3 months in patients undergoing MT for anterior circulation LVO beyond the conventional time window, compared to the control group. ANGEL-ASPECT, Study of Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core; BMM, best medical management; DAWN, Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo; DEFUSE-3, Endovascular Therapy Following Imaging Evaluation for Ischaemic Stroke-3; MR CLEAN LATE, endovascular treatment of acute ischaemic stroke in the Netherlands for late arrivals; mRS, modified Rankin Scale; MT, mechanical thrombectomy; RR, risk ratio; SELECT-2, Randomised Controlled Trial to Optimise Patient’s Selection for Endovascular Treatment in Acute Ischaemic Stroke; TENSION, The Efficacy and Safety of Thrombectomy in Stroke With Extended Lesion and Extended Time Window; TESLA, Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischaemic Stroke.
Forest plot of symptomatic intracranial haemorrhage rates in patients undergoing MT for anterior circulation LVO beyond the conventional time window, compared to the control group. ANGEL-ASPECT, Study of Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core; BMM, best medical management; DAWN, Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo; DEFUSE-3, Endovascular Therapy Following Imaging Evaluation for Ischaemic Stroke-3; MR CLEAN LATE, endovascular treatment of acute ischaemic stroke in the Netherlands for late arrivals; MT, mechanical thrombectomy; RR, risk ratio; SELECT-2, Randomised Controlled Trial to Optimise Patient’s Selection for Endovascular Treatment in Acute Ischaemic Stroke; TENSION, The Efficacy and Safety of Thrombectomy in Stroke With Extended Lesion and Extended Time Window; TESLA, Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischaemic Stroke.
Forest plot of mortality at 3 months in patients undergoing MT for anterior circulation LVO beyond the conventional time window, compared to the control group. ANGEL-ASPECT, Study of Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core; BMM, best medical management; DAWN, Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo; DEFUSE-3, Endovascular Therapy Following Imaging Evaluation for Ischaemic Stroke-3; MR CLEAN LATE, endovascular treatment of acute ischaemic stroke in the Netherlands for late arrivals; MT, mechanical thrombectomy; RR, risk ratio; SELECT-2, Randomised Controlled Trial to Optimise Patient’s Selection for Endovascular Treatment in Acute Ischaemic Stroke; TENSION, The Efficacy and Safety of Thrombectomy in Stroke With Extended Lesion and Extended Time Window; TESLA, Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischaemic Stroke.
Recommendation
Mechanical thrombectomy is recommended for patients with acute ischaemic stroke who present with National Institute of Health Stroke Scale ≥6 and Alberta Stroke Programme Early Computed Tomographic Score ≥3, with occlusions in the internal carotid artery or M1 within 24 hours of last known normal.
Level of evidence: A
Class of recommendation: I
In patients with anterior circulation LVO who met intravenous thrombolysis with alteplase or tenecteplase, does direct MT is non-inferior to bridging MT?
Analysis of current evidence
Six RCTs including Direct Endovascular Treatment vs Bridging Therapy for Patients with Acute Ischaemic Stroke (DEVT), Effect of Mechanical Thrombectomy Without versus With Intravenous Thrombolysis on Functional Outcome Among Patients With Acute Ischaemic Stroke (SKIP), Bridging Thrombolysis versus Direct Mechanical Thrombectomy in Acute Ischaemic Stroke, (SWIFT DIRECT), A Randomised Controlled Trial of DIRECT Endovascular Clot Retrieval vs Standard Bridging Thrombolysis With Endovascular Clot Retrieval (DIRECT-SAFE), Intravenous Treatment Followed by Intra-Arterial Treatment versus Direct Intra-Arterial Treatment for Acute Ischaemic Stroke Caused by A Proximal Intracranial Occlusion (MR CLEAN–NO IV), Direct Intra-arterial Thrombectomy in order to Revascularise Acute Ischemic Stroke Patients With Large Vessel Occlusion Efficiently in Chinese Tertiary Hospitals (DIRECT-MT) have completed to compare the outcomes between intravenous thrombolysis bridging MT and direct MT for LVO in the anterior circulation.54–59 DIRECT-MT and DEVT trials found direct MT is non-inferior to bridging MT for favourable functional outcome at 3 months (mRS 0–2), and other RCTs did not find positive results. The Improving Reperfusion Strategies in Acute Ischaemic Stroke (IRIS)60 trial which was the pool analysis of the individual patient data level from the six RCTs did not find direct MT is non-inferior bridging MT (90-day mRS: cOR 0.89, 95% CI 0.76 to 1.04 p=0.14).
Tenecteplase versus Alteplase Before Endovascular Therapy for Ischaemic Stroke (EXTEND-IA TNK)19 showed that among patients with confirmed LVO prior to planned MT, administering tenecteplase (0.25 mg/kg) within 4.5 hours of onset leads to better functional outcomes at 3 months (lower median mRS) compared with alteplase treatment, with no significant differences in safety outcomes between the two.
Recommendation
Patients with indications for mechanical thrombectomy (MT) should undergo treatment as soon as possible. When meeting the criteria for thrombolysis, we recommend that alteplase should be initiated first, while simultaneously considering bridging to MT.
Level of evidence: A
Class of recommendation: I
Patients with indications for MT should undergo treatment as soon as possible. When meeting the criteria for thrombolysis, we suggest that tenecteplase could be initiated first, while simultaneously considering bridging to MT.
Level of evidence: B
Class of recommendation: Ⅱa
In patients with ischaemic stroke due to basilar artery occlusion (BAO) of known onset time within 24 hours, does MT lead to better functional outcomes compared with BMM?
Analysis of current evidence
Currently, four large, prospective, multicentre RCTs demonstrated their results, including Acute Basilar Artery Occlusion: Endovascular Interventions vs Standard Medical Treatment (BEST), Basilar Artery International Cooperation Study (BASICS), Endovascular Treatment for Acute Basilar Artery Occlusion (ATTENTION) and Basilar Artery Occlusion Chinese Endovascular Trial (BAOCHE) trial.61–64 Among these studies, BEST (aOR 1.74, 95% CI 0.81 to 3.74) and BASICS (RR, 1.18, 95% CI 0.92 to 1.50) trials did not demonstrate the superiority of MT for achieving functional independent outcomes at 3 months (mRS 0–3) to BMM in patients with acute BAO within 8 hours or 6 hours of onset-to-puncture.61 63 In contrast, the ATTENTION trial (adjusted RR 2.06, 95% CI 1.46 to 2.91, p<0.01) and BAOCHE trial (adjusted RR 1.81, 95% CI 1.26 to 2.60; p<0.01) found that MT was significantly superior to BMM in achieving functional independence (mRS 0–3) at 3 months for patients with acute BAO treated within 12 hours or 6–24 hours of onset-to-puncture.62 64 The meta-analysis of the four RCTs also found that the functional independence outcomes of MT are superior to BMM for BAO (RR 1.54, 95% CI 1.16 to 2.04; p<0.01).65
Recommendation
For patients with acute basilar artery occlusion (BAO) within 12 h of onset that meet the inclusion criteria of the Endovascular Treatment for Acute Basilar Artery Occlusion and Basilar Artery Occlusion Chinese Endovascular Trial (BAOCHE) trials, mechanical thrombectomy (MT) is recommended.
Level of evidence: A
Class of recommendation: I
For patients with acute BAO between 12 hours and 24 hours of onset that meet the inclusion criteria of the BAOCHE trials, MT is reasonable.
Level of evidence: B
Class of recommendation: Ⅱa
Continuous quality improvement
The core of quality improvement in reperfusion therapy for acute ischaemic stroke lies in increasing reperfusion therapy rates and reducing waiting times for hospital treatment. What strategies, techniques or methods can help achieve continuous improvement in the quality of reperfusion therapy?
Current evidence
In the USA, Get with the Guidelines (GWTG) comprises a series of quality improvement programmes aimed at translating evidence-based guideline recommendations into clinical practice. Among them, the Target:Stroke programme initiative focuses on increasing reperfusion rates and reducing waiting times for hospital treatment for patients with acute ischaemic stroke.
Schwamm et al66 analysed data from the Target:Stroke programme’s continuous monitoring database and demonstrated that continuous quality improvement is effective in improving reperfusion therapy rates in ischaemic stroke (intravenous thrombolysis rate: 42.09% vs 72.84%). An array of interventions and improvement tools, including rapid triage and stroke team pre-notification, reduced the time from symptom onset to treatment, thereby improving clinical outcomes for patients.67 68 Researchers have also shown that implementing the Target:Stroke initiative resulted in a 109% increase in the proportion of patients receiving intravenous thrombolytic therapy with a door-to-needle time (DNT) of less than 60 min. Shortened DNT was associated with lower in-hospital mortality (9.93% vs 8.25%, adjusted OR 0.89, 95% CI 0.83 to 0.94, p<0.01), reduced sICH rates within 36 hours (5.68% vs 4.68%, adjusted OR, 0.83, 95% CI 0.76 to 0.91, p<0.01), and decreased rates of 1-year mortality or readmission (56.8% vs 53.1%, adjusted HR 1.08, 95% CI 1.05 to 1.10).69 70
Xian et al analysed large-scale stroke data from New York state and found that patients with acute ischaemic stroke treated at designated stroke centres had lower in-hospital mortality rates (10.1% vs 12.5%, adjusted mortality difference, −2.5%, 95% CI, −3.6% to −1.4%, p<0.01) and were more likely to receive intravenous thrombolysis (4.8% vs 1.7%, adjusted difference 2.2%, 95% CI 1.6% to 2.8%, p<0 .01).71 The Chinese Stroke Center Alliance, a project initiated by the CSA, aims to promote the establishment of stroke centres throughout China and improve the treatment efficiency of acute ischaemic stroke, particularly in reperfusion therapy. Analysis of large-scale data from the alliance found that the sustained stroke centres development has significantly increased intravenous thrombolysis rates (+60.3% relatively, 95% CI, 52.9% to 70.5%) and improved in-hospital outcomes for patients (in-hospital death or discharge against medical advice: −9.7% relatively, 95% CI −9.6% to −8.5%).72
In a cluster RCT in China, Professor Min Lou’s team demonstrated that a six-factor intervention model based on the ‘Behavior Change Wheel’ framework with monthly remote teleconferencing training-significantly reduced the time to initiate intravenous thrombolysis (the proportion of DNT≤60 min: 82.0% vs 73.3%, adjusted OR 1.77, 95% CI 1.17 to 2.70, p<0.01) and improved clinical outcomes for patients with ischaemic stroke (mRS 0–1: adjusted OR 1.38, 95% CI 1.00 to 1.90, p=0.049).73
Professor Yongjun Wang’s team, through a stepped-wedge cluster randomised controlled Study—Effectiveness of a Quality Improvement Intervention on Reperfusion Treatment for Patients with Acute Ischaemic Stroke (IMPROVE)—validated the effectiveness of STEP intervention (S: strategies, T: toolkit, E: exploration, P: paradigm). These interventions, grounded in implementational science and focused on problem-oriented, targeted remote guidance and continuous feedback, showed a trend of increasing intravenous thrombolysis and endovascular therapy rates, particularly in secondary hospitals (57.8% vs 42.9%, adjusted OR, 2.24, 95% CI 1.29 to 3.88).74 Currently, Wang’s team is also conducting research on an emergency stroke unit based on low-field mobile MRI technology, which is expected to further reduce waiting times for hospital treatment and increase the reperfusion therapy rate for patients with acute ischaemic stroke.
Recommendation
Continuous quality improvement using effective tools is a proven strategy for increasing reperfusion therapy rates and improving clinical outcomes for patients with acute ischaemic stroke.
Level of evidence: B
Class of recommendation: Ⅱa
Establishing database-driven continuous monitoring and feedback systems for the reperfusion therapy process is a crucial tool for quality improvement in ischaemic stroke care.
Level of evidence: B
Class of recommendation: Ⅱa
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Footnotes
X @hqgu
Collaborators Chinese Stroke Association Guidelines Writing Group on Reperfusion Therapy for Acute Ischemic Stroke (Sorted in A-Z order): Anding Xu, Department of Neurology, The First Affiliated Hospital of Jinan University, Guangzhou, Guangdong, China; Aoming Jin, China National Clinical Research Center for Neurological Diseases, Beijing, China; Benyan Luo, Department of Neurology, the First Affiliated Hospital of Zhejiang University, Hangzhou, China; Bin Peng, Department of Neurology, Peking Union Medical College Hospital, Beijing, China; Bo Hu, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Caiyun Wang, China National Clinical Research Center for Neurological Diseases, Beijing, China; Chao Qin, Department of Neurology, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi Province, China; Chunjuan Wang, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Dapeng Sun, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Dehong Lu, Department of Pathology, Xuanwu Hospital, Capital Medical University, Beijing, China; Dou Li, Beijing Emergency Medical Center, Beijing, China; Gang Zhao, Department of Neurology, Xijing Hospital, Fourth Military Medical University, Xi’an, China; Fudong Shi, Department of Neurology, Institute of Neuroimmunology, Tianjin Medical University General Hospital, Tianjin, China; Hongqiu Gu, China National Clinical Research Center for Neurological Diseases, Beijing, China; Jianmin Zhang, Department of Neurosurgery, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China; Jianxin Zhou, Department of Intensive Care Unit, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Jinsheng Zeng, Department of Neurology, The First Affiliated Hospital, Sun Yat-Sen University; Guangdong Provincial Key Laboratory for Diagnosis and Treatment of Major Neurological Diseases, National Key Clinical Department and Key Discipline of Neurology, Guangzhou, China; Jiong Shi, Department of Neurology, Institute on Aging and Brain Disorders, the First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China; Junbo Ge, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, Shanghai, China; Jun Pu, Kunming Medical University, Kunming, China; Kaifu Ke, Department of Neurology, Affiliated Hospital of Nantong University, Nantong, China; Kai Wang, Department of Neurology, the First Affiliated Hospital of Anhui Medical University, China; Lan Chu, Department of Neurology, the Affiliated Hospital of Guizhou Medical University, Guiyang, China; Li He, Department of Neurology, West China Hospital of Sichuan University, Chengdu, China; Li Guo, Department of Neurology, The Second Hospital of Hebei Medical University, Shijiazhuang, China; Lihua Wang, Department of Neurology, the Second Affiliated Hospital of Harbin Medical University, Harbin, China; Ling Yin, Department of Neurology, Chinese People’s Liberation Army General Hospital, Beijing, China; Linong Ji, Department of Endocrinology, Peking University People’s Hospital, Xicheng District, Beijing, China; Liping Liu, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Ning Wang, Department of Neurology and Institute of Neurology, First Affiliated Hospital, Fujian Medical University, Fuzhou, China; Qiang Dong, Department of Neurology, Huashan Hospital, Fudan University, Shanghai, China; Shengnian Zhou, Department of Neurology, Qilu Hospital of Shandong University and Brain Science Research Institute, Shandong University, Jinan, Shandong, China; Shuo Wang, Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Shuya Li, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Tong Zhang, Neurorehabilitation Department, China Rehabilitation Research Center, Beijing, China; Weixin Cai, Nursing Department, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Wenzhi Wang, Department of Neuroepidemiology, Beijing Neurosurgical Institute, Capital Medical University, Beijing, China; Xiaomu Wu, Department of Neurology, Jiangxi Provincial People’s Hospital, Nanchang, Jiangxi, China; Xiaoning Zhang, Department of Neurology, First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang Uygur Autonomous Region, China; Xiaoping Gao, Department of Neurology, The First Affiliated Hospital of Hunan Normal University, Changsha, China; Xiaoyuan Niu, Department of Neurology, First Hospital of Shanxi Medical University, Taiyuan, Shanxi Province, China; Xingquan Zhao, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Xin Wang, Department of Neurology, Zhongshan Hospital, Fudan University, Shanghai, China; Xunming Ji, Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China; Yanhui Du, Department of Neurology, General Hospital, Ningxia Medical University, Yinchuan, 750004, China; Yilong Wang, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Yi Yang, Department of Neurology, the First Bethune Hospital of Jilin University, Changchun, China; Yong Huo, Department of Cardiology, Peking University First Hospital, Beijing, China; Yongjun Wang, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Yuming Xu, Department of Neurology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China; Yun Xu, Department of Neurology, Drum Tower Hospital Affiliated to Nanjing University School of Medicine, China; Yunyun Xiong, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Zhengqi Lu, Department of Neurology, Mental and Neurological Disease Research Center, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China; Zhong Chen, Department of Vascular Surgery, Capital Medical University Affiliated Beijing Anzhen Hospital, Beijing, China; Zhiyi He, Department of Neurology, The First Affiliated Hospital of China Medical University, Shenyang, China; Zhongrong Miao, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, China; Zixiao Li, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Contributors YX, SL, CW, DS and ZL were responsible for drafting and revising the manuscript. HG and AJ provided methodological and statistical guidance. QD, LL, ZM and YW reviewed and verified the guideline recommendations and determined the recommendation level for each piece of evidence.
Funding Beijing Outstanding Young Scientist Program (JQ24058); Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0505400, 2023ZD0505401, 2023ZD0505402, 2023ZD0505404)
Competing interests None declared.
Provenance and peer review Commissioned; internally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.