Abstract
Background Dual-energy CT (DECT) provides several novel methods to assess thrombus perviousness. We aimed to evaluate whether the novel thrombus perviousness measured with DECT is associated with improved recanalisation and better functional outcomes in acute ischaemic stroke (AIS) patients with endovascular thrombectomy (EVT).
Methods 108 AIS patients with middle cerebral artery occlusion who underwent DECT angiography on admission and received EVT treatment between April 2020 and September 2023 were retrospectively analysed. Thrombus attenuation increase (TAI) was evaluated on routine CT angiography and non-contrast CT, and DECT quantitative parameters of thrombus, including iodine concentration (IC) and normalised IC (NIC) were measured. Multivariable logistic regression analysis was used to evaluate the association of thrombus characteristics with arterial occlusive lesion scale and 90-day modified Rankin Scale.
Results NIC was significantly associated with successful recanalisation (OR 1.372 (95% CI 1.194 to 1.625); p<0.001) and good functional outcome (OR 1.252 (95% CI 1.114 to 1.446); p<0.001). NIC yielded higher performance, with area under curve (AUC) of 0.789 and 0.740, in the prediction of recanalisation and functional outcome than TAI (AUCs=0.635 and 0.592). Compared with low-level NIC thrombus, high-level NIC was associated with 11.4 and 15.4 times higher likelihood of successful recanalisation and good functional outcome. Moreover, NIC was a significant indicator to differentiate large artery atherosclerosis from cardioembolism stroke with high specificity and positive predictive value.
Conclusions Higher DECT-derived NIC is associated with increased odds of successful recanalisation and good functional outcome for EVT patients, and it yielded higher prediction performance than TAI.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Previous studies have investigated that thrombus perviousness is associated with the recanalisation and outcomes of endovascular thrombectomy (EVT). Dual-energy CT (DECT) could provide several novel methods to assess thrombus perviousness.
WHAT THIS STUDY ADDS
This study revealed that compared with thrombus attenuation increase (TAI), normalised iodine concentration (NIC) derived from DECT could provide higher prediction performance on recanalisation and outcomes in EVT patients. NIC was also associated with the origin of thrombus.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These findings emphasise the significance of iodine-based thrombus perviousness, and it provides higher predictive performance than TAI for recanalisation status and clinical outcomes in patients with EVT treatment. It may lead to a new imaging marker to aid in device selection and treatment decisions in EVT patients.
Introduction
Endovascular thrombectomy (EVT) has been established as the preferred treatment in the initial phase of acute ischaemic stroke (AIS) caused by anterior large vessel occlusion (LVO) after the release of a recent pivotal trial.1–4 Effective recanalisation significantly impacts the clinical outcomes of patients treated with EVT. Meanwhile, recent studies have suggested that the composition of the thrombus could affect the difficulty of EVT treatment and functional outcome, as thrombus is highly heterogeneous.5 6 As a result, accurately identifying thrombus characteristics on baseline imaging findings would substantially help neurointerventional radiologists plan procedures and make treatment decisions.
Thrombus perviousness refers to the ability of the blood to penetrate irregular gaps within the thrombus,7–9 and it is quantitatively evaluated by comparing the CT value between non-contrast CT (NCCT) and routine CT angiography (CTA). In previous studies, perviousness obtained by this approach, such as thrombus attenuation increase (TAI) and void fraction, was associated with recanalisation and functional outcome in patients undergoing intravenous thrombolysis (IVT).10 11 However, the effects of perviousness vary significantly among endovascular treatment patients.12–15
Currently, dual-energy CT (DECT) has proven to be a valuable tool for characterising materials according to their differential attenuation characteristics at different electron volts, and it offers an interesting approach to facilitate absolute iodine quantification based on the multimaterial decomposition algorithm,16 17 making DECT widely used in clinical practice.18–20 During DECT angiography examination, the quantity of iodine contrast agent in the thrombus correlates positively with perviousness, and DECT allows direct acquisition of various thrombus quantitative parameters. Recent research has suggested that iodine-based perviousness derived from DECT overlay maps is more accurate in predicting the functional outcomes of patients undergoing IVT compared with conventional CT.21 However, to our knowledge, it has not yet been investigated how thrombus iodine-based perviousness would reflect the recanalisation and predict the functional outcomes of patients after EVT treatment.
The purpose of our study was to investigate the accuracy of thrombus iodine-based perviousness in differentiating recanalisation and functional outcomes of patients with anterior circulation AIS after EVT. Second, we also aim to determine the association between thrombus DECT quantitative parameters and stroke cause.
Methods
Patients
This article follows the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.22
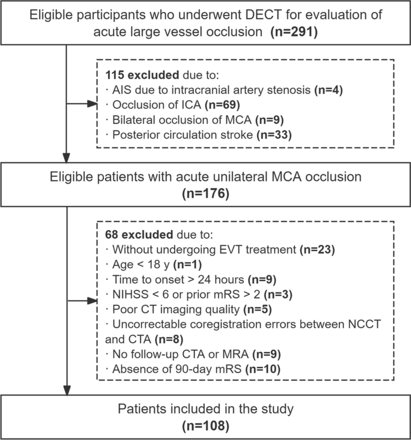
Between April 2020 and September 2023, a total of 291 patients suspected of acute LVO who underwent baseline NCCT and DECT angiography at Tianjin Huanhu Hospital were retrospectively enrolled in the study. The inclusion criteria were (a) underwent EVT treatment; (b) age ≥18 years; (c) time to onset <24 hours and (d) AIS due to unilateral M1 or proximal M2 segment of middle cerebral artery (MCA) occlusion. Exclusion criteria were (a) poor imaging quality; (b) occlusion combined with ipsilateral extracranial internal carotid artery (ICA) occlusion; (c) bilateral occlusion of ICA or MCA; (d) uncorrectable coregistration errors between NCCT and CTA and (e) no follow-up CTA or MR angiography (MRA) was performed within 72 hours (±24 hours) after EVT, (f) National Institutes of Health Stroke Scale (NIHSS) <6 or a prior modified Rankin Scale (mRS) of >2 and (g) the absence of 90-day follow-up. Finally, 108 patients were enrolled in this study. Figure 1 provides a detailed flow chart of the inclusion and exclusion criteria.
Study flow chart. AIS indicates acute ischaemic stroke; CTA, CT angiography; DECT, dual-energy CT; ICA, internal carotid artery; EVT, endovascular thrombectomy; MCA, middle cerebral artery; MRA, MR angiography; mRS, modified Rankin Scale; NCCT, non-contrast CT; NIHSS, National Institutes of Health Stroke Scale.
Imaging protocols
Patients were scanned with 256-slice multidetector CT scanners (Revolution CT; GE HealthCare, Chicago, USA) with the build-in gemstone spectral imaging technology (GSI, GE HealthCare) by switching the tube voltage between 80 and 140 peak kilovoltage (kVp) in 0.25 ms. The protocol for each patient included NCCT, 70-keV monochromatic CTA and GSI DECT angiography. After scanning, the above axial image sets with a slice thickness of 1.25 mm were reconstructed and transferred to an offline workstation (GE Advantage Workstation AW4.7, GE Healthcare). Detailed imaging parameters are provided in online supplemental methods.
Supplementary data
Thrombus perviousness measurements
All baseline imaging postprocessing and assessments were performed by two neuroradiologists (SL and CT) with 7 and 23 years of experience in interpreting acute stroke data, respectively. Except for the symptomatic side, readers were blinded to all clinical data, discrepancies in scores were settled by consensus discussion and the quantitative perviousness parameters were averaged across two readers.
NCCT and 70-keV monochromatic CTA derived from DECT angiography were used to calculate TAI, which is defined as routine thrombus perviousness as described previously.11 NCCT and 70-keV monochromatic CTA images were coregistered using rigid registration with Compare software (GE Healthcare) on the AW4.7 Workstation. In the case of suboptimal coregistration, manual corrections were performed using the 3D Slicer (V.5.6.0; https://www.slicer.org).23 The detailed measurement of thrombus localisation and extension are shown in online supplemental methods and online supplemental figure S1. Three spherical regions of interest (ROIs) with a radius of 1 mm were placed within the proximal, middle and distal parts of the thrombus, and average density (ρ in Hounsfield units (HU)) of all ROIs was calculated for NCCT and monochromatic CTA.12 15 TAI was calculated according to the following formula: TAI = ρVM-CTA–ρNCCT.
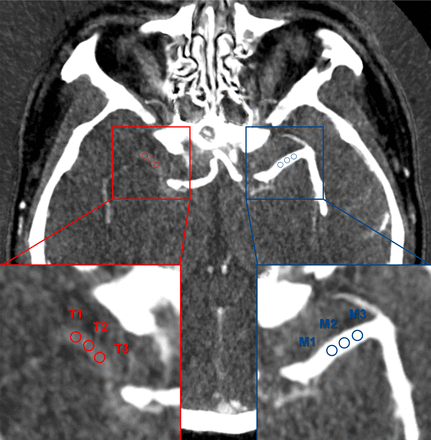
Thrombus iodine-based quantitative parameters were extracted from DECT images using GSI Viewer software (GE Healthcare). On the iodine overlap images with a thickness of 2.5 mm, three circular ROIs with a radius of 1 mm were placed in the proximal, middle and distal parts of the thrombus. In the event of a small thrombus, the ROIs may partially overlap. The mean value of three ROIs was recorded as iodine concentration (IC) of thrombus. As a reference, the IC of the contralateral MCA (ICMCA) was also measured. The normalised IC (NIC) was calculated as the value of ICthrombus divided by ICMCA (figure 2). The formula for the calculation was as follows:
NICthrombus=ICthrombus/ICMCA
Normalised iodine concentrations (NIC) measurement. Thrombus iodine concentrations (ICs) were measured on iodine overlay maps derived from dual-energy CT. The NIC was calculated as IC of thrombus divided by IC of contralateral MCA. MCA, middle cerebral artery.
All the ROIs were automatically copied onto all monochromatic and effective atomic number (Zeff) images. CT values at 40 keV and 70 keV in the monochromatic images, as well as the Zeff values, were measured within the ROIs, and the average of these three ROIs was calculated. The λHU was calculated as follows: λHU=(attenuation value of the thrombus in 40 keV–attenuation value of the thrombus in 70 keV)/30.24 Assessments of other imaging characteristics are detailed inonline supplemental methods.
Clinical variables and study outcomes
Baseline clinical data were collected, including the following variables: (a) demographic information, such as age, sex and the interval time from symptom onset to admission imaging; (b) NIHSS; (c) cerebrovascular risk factors, including hypertension, diabetes mellitus, hyperlipidaemia, history of stroke or transient ischaemic attacks, coronary artery disease, atrial fibrillation, smoking and alcohol consumption and (d) the ischaemic stroke cause, according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification.25 The prestroke mRS was used to assess the level of function before the stroke, as detailed in previous studies (see online supplemental methods).26 The modified Thrombolysis Score for Cerebral Infarction (mTICI) was used to evaluate reperfusion after thrombectomy.
The primary outcome was successful recanalisation, defined as an arterial occlusive lesion (AOL) scale score of 2 or 3. The AOL scale was assessed on the follow-up CTA or MRA within 72±24 hours after EVT. We included the 90-day mRS to indicate functional outcomes. The mRS is a 7-point scale, ranging from 0 (no symptoms) to 6 (death). A good functional outcome was defined as an mRS score of 0–2.
Based on routine workups throughout the hospital stay following acute admission (such as 24-hour rhythm monitoring, Doppler sonography of the cervical arteries and echocardiography), the in-hospital treating physician determined the stroke cause according to TOAST classification. AOL scale score was evaluated by a neuroradiologist (SL and LF) with 7 and 15 years of experience in neuroimaging. And, the NIHSS, mTICI and mRS scores were assessed by two neurologists (LL and SW) with 10 and 17 years of experience, respectively, who were blinded to the CT imaging. In cases of disagreement among the readers, we consulted an additional neurologist with 21 years of experience to make the final decision.
Statistical analysis
Normality testing was performed to assess the variable distribution. Univariate analysis (χ2 test or Fisher’s exact test for comparing the categorical variables, independent t-test or Mann-Whitney U test for continuous variables) was used to assess the association between outcomes and thrombus characteristics. Afterwards, a multivariate logistic regression analysis was performed, including variables with p<0.10 in the univariate analysis. Three logistic regression models were fitted to estimate ORs for the association between thrombus characteristics and outcomes, along with their corresponding 95% CIs. Models 1 and 2 were multivariate models for successful recanalisation, including TAI and NIC, respectively, while models 3 and 4 focused on functional outcomes, also incorporating TAI and NIC, respectively. NIC was categorised into three levels on the basis of its tertiles (<0.126, 0.126–0.163 and >0.163), and trend tests were conducted using the median value of each tertile. To investigate the relationship between NIC and outcome occurrence, restricted cubic spline (RCS) based on logistic regression analysis was employed, and the NIC value at OR=1 was treated as the reference. Receiver operator characteristic (ROC) curve analyses were performed to assess the discriminatory power of the variables and models in predicting the recanalisation and functional outcome. Area under curve (AUC), sensitivity, specificity, positive predictive value, negative predictive value and accuracy were used to assess model performance. Comparison of the AUC differences between the variables was evaluated using the two-sided DeLong’s test. Kruskal-Wallis H test with Bonferroni correction and post hoc analysis was used to evaluate the difference in thrombus characteristics in TOAST subtypes. A two-tailed p<0.05 was considered significant for all statistical tests. All statistical analyses were performed using R language (V.4.3.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients characteristics
In total, 108 patients who underwent EVT were included in the analysis. The mean age of the patients was 61.44±10.74 years, and 89 (82.4%) were men. The median admission NIHSS was 13 (IQR 10–15), and the median baseline Alberta Stroke Programme Early CT Score (ASPECTS) was 7 (IQR 5–8). The median time from symptom onset to admission imaging was 354 min (IQR 239–530 min). The distribution of NIC based on AOL and mRS scale is illustrated in online supplemental figure S2. Results concerning the consistency of thrombus characteristics, AOL and mTICI assessments, as well as the association between TAI and thrombus iodine-based parameters, are presented in online supplemental figure S3-S7.
Thrombus iodine-based perviousness and successful recanalisation
Final AOL score 2–3 was achieved in 70 (64.81%) patients. Comparisons of clinical and imaging characteristics between the successful recanalisation group and unsuccessful recanalisation group are listed in table 1.
Baseline, imaging and treatment characteristics compared by recanalisation status and functional outcome
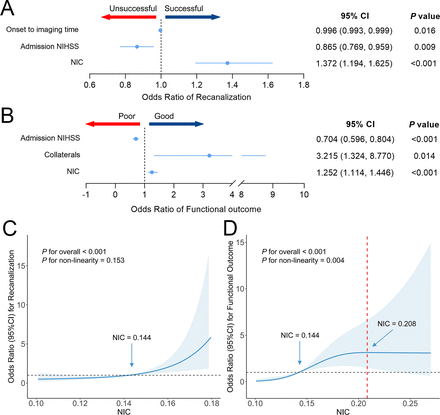
In multivariable regression analysis, time from symptom onset to imaging (OR 0.996 (95% CI 0.993 to 0.999); p=0.016), admission NIHSS (OR 0.865 (95% CI 0.769 to 0.959); p=0.009) and NIC (OR 1.372 (95% CI 1.194 to 1.625); p<0.001) were significantly associated with successful recanalisation after adjusting for age, sex and baseline ASPECTS (figure 3A). In the trend test, RCS curve suggested a significant and linear relationship between NIC and recanalisation after adjusting for covariates (p for overall <0.001 and p for non-linearity=0.153). The adjusted OR of NIC increased significantly with a higher likelihood of successful recanalisation, and the nadir point was around 0.144. After adjusting for covariates, moderate-level NIC (0.126–0.163) was associated with a 1.9 times greater likelihood of successful recanalisation than low-level NIC (<0.126) and 11.4 times increase for high-level NIC (>0.163) (p for trend <0.001). The detailed results are shown in table 2 and figure 3C.
Forest plots and restricted cubic splines (RCS) for recanalisation and functional outcome. (A, B) Dots depict the ORs, and horizontal bars depict 95% CI. ORs are indicated by solid lines, and 95% CIs by shaded areas in RCS. The model for predicting successful recanalisation (C) was adjusted for time from symptom onset to imaging and admission NIHSS, and the model for predicting good functional outcome (D) was adjusted by admission NIHSS and collaterals. NIC, normalised iodine concentration; NIHSS, National Institutes of Health Stroke Scale.
Linear trend test of NIC for recanalisation and functional outcome
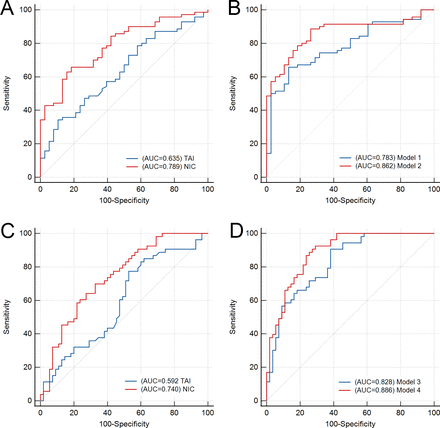
In ROC analysis, NIC achieved the highest AUC of 0.789 (95% CI 0.704 to 0.874), and it was significantly higher than the conventional perviousness parameter TAI (AUC 0.635 (95% CI 0.528 to 0.742)) (Z=−2.563, p=0.010 for the comparison) in the prediction of recanalisation. These results are detailed in table 3 and figure 4A. NIC provided higher sensitivity in predicting successful recanalisation compared with TAI, with 65.71% (46/70) vs 34.29% (24/70), respectively (χ2=13.829, p<0.001). Furthermore, the accuracy of NIC was slightly higher than that of TAI, at 71.30% (77/108) compared with 53.70% (58/108) (χ2=7.131, p=0.011), as shown in online supplemental table S1. Model 1, which incorporated TAI and clinical parameters (time from symptom onset to imaging and admission NIHSS), achieved an AUC of 0.783 (95% CI 0.696 to 0.870). Model 2, combining NIC, time from symptom onset to imaging and admission NIHSS, demonstrated a superior AUC of 0.862 ((95% CI 0.791 to 0.932)) compared with model 1 (Z=2.125, p=0.034 for comparison), as detailed in figure 4B and online supplemental table S2.
Receiver operating characteristic (ROC) curve analysis for successful recanalisation and good functional outcome. ROC curve analysis for normalised iodine concentration (NIC) and thrombus attenuation increase (TAI) (A, C), and multivariable prediction models (B, D). AUC, area under the curve.
Receiver operating characteristic curve analysis to classify recanalisation and functional outcome
Thrombus iodine-based perviousness and functional outcome
According to the 90-day mRS score, 53 patients (49.07%) achieved a good functional outcome, while 55 patients (50.93%) had a poor functional outcome. The detailed results of the comparison between successful recanalisation group and unsuccessful recanalisation group are listed in table 1.
Lower admission NIHSS (OR 0.704 (95% CI 0.596 to 0.804); p<0.001), better collateral score (OR 3.215 (95% CI 1.324 to 8.770); p=0.014) and higher NIC (OR 1.252 (95% CI 1.114 to 1.446); p<0.001) were positively associated with a greater likelihood of a good functional outcome in multivariable regression analysis, adjusting for age, sex, baseline ASPECTS and prestroke mRS (figure 3B). The RCS curve showed that the adjusted ORs of good functional outcome increased with higher NIC, with the nadir point around 0.144 (figure 3D). The probability of a good functional outcome was increasing until NIC reached approximately 0.208 (OR 3.137 (95% CI 1.485 to 6.628)), after which it began to decrease (p for overall <0.001 and p for non-linearity=0.004).
The AUC of NIC was 0.740 (95% CI 0.647 to 0.833), which was higher than that of TAI (AUC 0.592 (95% CI 0.483 to 0.708)) in predicting good functional outcome (Z=−2.661, p=0.008 for the comparison) (table 3 and figure 4C). Although the sensitivity of NIC was slightly lower than that of TAI (69.81% (37/53) vs 77.36% (41/53); χ2=0.857, p=0.439), the specificity of NIC was slightly higher than that of TAI (67.27% (37/55) vs 47.27% (26/55)), p=0.053). Additionally, the multivariable models, model 3 and model 4 achieved AUCs of 0.828 (model 3; 95% CI 0.752 to 0.904) and 0.886 (model 4; 95% CI 0.825 to 0.947), respectively, in differentiating functional outcome (figure 4D and online supplemental tables S1, S2). The probability curves and optimal cut-off value of NIC for predicting both recanalisation and functional outcomes are detailed in online supplemental figure S8 and table S3.
Association between NIC and stroke cause
64 (59.26%) patients experienced a large artery atherosclerosis (LAA) (TOAST category 1), whereas 33 (30.56%) patients suffered a cardioembolism stroke (CE) (TOAST category 2). 11 patients (10.19%) had a stroke of undetermined cause (TOAST category 5). No patients had a stroke due to small vessel or penetrating artery disease (TOAST category 3) or stroke of other determined cause (TOAST category 4).
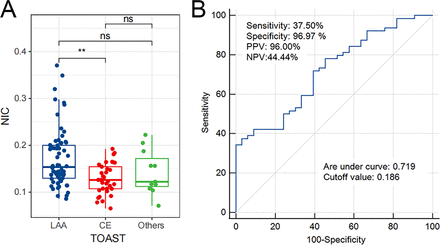
NIC was significantly associated with stroke subtypes according to the TOAST classification (p=0.002). In post hoc pairwise comparisons, NIC was significantly higher in LAA compared with CE stroke (median, 15.357 (12.957, 20.034) vs 12.608 (10.570, 15.655); p=0.001 after Bonferroni adjustment). Additionally, ROC analysis indicated that NIC is significant indicator to identify LAA (LAA vs CE), with an AUC of 0.719 (95% CI 0.616 to 0.823). The optimal NIC cut-off value for categorising a thrombus as LAA was 0.186, with a specificity of 96.97% and a positive predictive value of 96.00%. An NIC cut-off value of >0.193 achieved 100% specificity (at 34.38% sensitivity). The distribution of NIC and the ROC curve according to stroke cause is presented in figure 5.
Box-and-whisker plots and receiver operating characteristic (ROC) curve of the association between normalised iodine concentration (NIC) and stroke cause. (A) NIC was associated with stroke cause (Kruskal-Wallis test; p=0.002). Post hoc pairwise comparison between large artery atherosclerosis stroke (LAA) and cardioembolic (CE) stroke showed significant difference (p=0.001 after Bonferroni adjustment). (B) ROC curve demonstrated the ability of NIC to distinguish LAA from CE strokes. The optimal NIC cut-off value to categorise a thrombus as LAA was 0.186, with a specificity of 96.97% and a positive predictive value of 96.00%. NPV, negative predictive value; PPV, positive predictive value; TOAST, Trial of ORG 10172 in Acute Stroke Treatment. **p<0.01.
Discussion
The importance of thrombus characteristics is increasingly acknowledged in predicting of recanalisation and clinical outcomes in patients undergoing EVT.21 In this study, we attempted to evaluate the impact of DECT quantitative parameters of thrombus on recanalisation and functional outcome after EVT. We demonstrated that higher NIC was associated with a high probability of the successful recanalisation and good functional outcome. NIC showed better predictive performance than TAI for both recanalisation and functional outcomes. Our results also suggested that an NIC around 0.208 provided the highest resolution capability in identifying functional outcomes. Additionally, subgroup analysis based on the TOAST classification revealed that patients with LAA stroke had higher NIC than those with CE stroke.
Perviousness based on NCCT and conventional CTA is routinely used to estimate potential contrast agent penetration of the thrombus. In previous studies, hyperdense thrombus and TAI were the most common traditional indicators of thrombus perviousness.7 27 The association between these traditional indicators and successful recanalisation, as well as good functional outcomes, has been fully investigated in IVT patients.10 11 But conflicting conclusions regarding the relationship between these indicators and outcomes were observed in EVT patients. Several studies have shown that patients with higher hyperdense thrombus have a greater likelihood of successful recanalisation and improved functional outcome.13–15 However, the opposite conclusions were dropped by Kappelhof et al.12 There are several possible explanations for these discrepancies: First, the time interval between symptom onset and EVT treatment is typically longer than that for IVT, and EVT is generally more successful in achieving recanalisation compared with IVT. This may lead to significant differences in the association between thrombus perviousness and recanalisation, as well as functional outcomes after EVT and IVT. Second, thrombus density measured via NCCT and conventional CTA is an indirect measurement of IC of contrast agent in thrombus. Additionally, coregistration errors in TAI measurement are inevitable, potentially leading to significant inaccuracies in thrombus perviousness parameters.
DECT enables direct quantification of the IC in lesions using material decomposition technique. Several studies have demonstrated a strong correlation between IC levels and iodine contrast content in voxels.18 28 Some studies have also verified the association between DECT-derived parameters and thrombus composition.24 29 30 Dai et al proposed measurements of thrombus perviousness based on DECT, with their results showing that NIC could predict recanalisation and prognosis after IVT.21 However, to the best of our knowledge, no studies have explored the relationship between thrombus IC and recanalisation or functional outcome in EVT patients. Our results indicated that NIC is an independent predictor of recanalisation in EVT patients, exhibiting the highest sensitivity and accuracy among all predictors. Compared with the conventional predictor, TAI generated with traditional CT, NIC showed significantly higher AUC and sensitivity in identifying recanalisation status. These results can be explained by the following: (1) DECT quantitative parameters more accurately reflect the actual iodine content in the thrombus, and NIC diminishes the influence of native thrombus density and arterial wall structure,24 31 making it a more sensitive indicator of thrombus perviousness than TAI; (2) NIC values are measured from iodine overlay maps, eliminating the need for coregistration, and thereby avoiding error association with this process. Thus, thrombus iodine-based characteristics offer significant clinical value as a novel imaging biomarker for the recanalisation in EVT.
Similar to the results in IVT patients, we confirmed the association between higher NIC and improved functional outcome in EVT patients. This can be explained by the hypothesis that a more permeable thrombus allows blood to pass through, thereby enhancing oxygenation of ischaemic cerebral tissue distal to the occlusion. However, we noticed conflicting conclusions regarding the correlation between TAI and functional outcome in previous studies,12 13 In our results, the association between TAI and 90-day mRS was weak. The instability of TAI measurement could contribute to these discrepancies.8 Despite a high AUC, the specificity and positive predictive value of NIC in predicting functional outcomes were only moderate in our study. This suggests that NIC should be considered alongside clinical factors in clinical decision-making.
Moreover, patients with LAA stroke were more likely to have higher NIC values compared with those with other stroke causes. NIC demonstrated greatly high specificity and positive predictive value in distinguishing LAA from CE stroke. Histopathological studies have indicated that red blood cells (RBC)-rich thrombus is significantly associated with noncardioembolic stroke.32 33 We speculated that thrombus with high NIC is likely to be rich in RBCs. Fibrin-rich thrombus typically exhibit dense fibrin deposition, while RBC-rich thrombus is encircled by a thin fibrin network.34 The higher NIC value in RBC-rich thrombus may be attributed to its physical characteristics, which allow greater penetration of blood and contrast agents. Additionally, RBC-rich thrombus exhibits decreased stiffness and increased deformability,35 potentially leading to more effective recanalisation outcomes. The association between high NIC, higher rates of successful recanalisation and improved functional outcomes further supports this hypothesis. Thus, a higher NIC could indicate greater permeability to iodinated contrast agent within the thrombus, a softer texture and increased suitability for thrombectomy. Given its high specificity and positive predictive value, NIC holds considerable potential in determining the causes of occlusions, especially in intracranial atherosclerotic occlusions. This could influence the choice of stenting implantation or sole thrombectomy, as well as the requirement for postprocedural antiplatelet therapy. Based on our results, the NIC level could provide guiding value for the treatment strategies in clinical practice. For thrombus with high NIC, standard stent retriever or aspiration are suitable. To minimise the risk of peri-interventional thrombus fragmentation and subsequent downstream embolism,36 the use of proximal balloon catheters is recommended. Given the stiffness and higher friction coefficient characteristic of low NIC thrombus (fibrin-rich), employing intermediate catheters or larger bore aspiration catheters can enhance the success rate of thrombectomy procedures.
This study has several limitations. First, the single-centre retrospective design may have resulted in selection bias. Second, the sample size was relatively small since not all patients receiving EVT followed the DECT protocol in their preoperative evaluation, limiting the generalisability of our findings. Third, our analysis was confined to patients with M1 and proximal M2 segment of the MCA, excluding those with concomitant ipsilateral ICA involvement. Further studies are needed to assess whether our results can be generalised to patients with ICA or basilar artery occlusion. Fourth, the AOL scale was used to evaluate the revascularisation status, as opposed to the mTICI. Future studies should integrate both recanalisation and reperfusion metrics to enable a more comprehensive understanding of how thrombus characteristics influence patient’s outcomes. Fifth, pathological analysis of the thrombus was not performed in this study, and further research is required to explore the association between thrombus IC and its pathological composition. Sixth, the proportion of patients who received IVT before EVT is low, preventing the investigation of the impact of IVT on thrombus DECT characteristics and its interaction with recanalisation outcomes, and it will be an interesting topic for future research. Furthermore, our study was based on a single-type DECT scanner. While we believe our finding could be applicable to other DECT scanners, the optimal cut-off values may vary across different scanner models, particularly those built on different implementation principles.
Conclusions
In conclusion, NIC assessed through DECT imaging offered a novel and more accurate method for evaluating thrombus perviousness compared with TAI in predicting recanalisation and functional outcome after EVT treatment. Additionally, NIC could provide high specificity and positive predictive value in differentiating LAA from CE stroke. Our findings suggested that iodine content within thrombus, as measured by DECT, could serve as a valuable imaging biomarker to guide device selection and treatment decisions in EVT patients. Further prospective, larger-scale, multicentre studies are required to validate these findings.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and the study’s design and protocol was approved by the Ethic Committee of Tianjin Huanhu Hospital (Clinical Study Approval 2022-072). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors thank all radiographers and nurses, Radiology department of Tianjin Huanhu Hospital, for image acquisition; Bohao Zhang, MD, Neurosurgery department of Tianjin Huanhu Hospital, for patient recruitment.
Footnotes
CT and SL contributed equally.
Contributors CT, SL, NH and TH conceived and designed the study. CT and SL contributed to clinical data collection, sample collection and wrote the manuscript. LF, JG and CC carried out the majority of computational analyses and drafting of tables and figures. YS, TR and HW contributed to data analysis. SW and LL performed the operation and clinical data collection. LW, MW, SX, SJ and TH revised the manuscript and interpreted findings. All authors reviewed and approved the final version of the manuscript. NH and TH, who acting as guarantors, provided overall oversight of the research.
Funding This work was sponsored by Tianjin Health Research Project (Grant No. TJWJ2022QN061 to SL), Tianjin Medical Talent Project (Grant No. TJSJMYXYC-D2-059 to TH) and Tianjin Municipal Health Commission Special Plan for High Level Talents in Science and Technology Project (Grant No.TJWJ2024RC016 to TH).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.