Abstract
Background and purpose Approximately 25% of acute large vessel occlusive (LVO) ischaemic strokes are of unknown thrombotic origin, and there is a need to establish the aetiology to guide subsequent preventative measures. The aim of this study was to quantify thrombus composition in patients with LVO and explore associations between thrombus composition and stroke aetiology.
Methods Thrombi were extracted from 132 patients with acute ischaemic stroke. Erythrocytes, leucocytes and F+P (fibrin+platelet) proportions were assessed in tissue sections stained with H&E, while CD3+ T cells and neutrophil extracellular traps (NETs) were quantified in immunohistochemistry-stained sections. Thrombus components, clinical parameters and interventional variables were compared between different stroke subtypes defined by Trial of ORG 10172 in Acute Stroke Treatment criteria.
Results F+P composition was significantly higher (p<0.001) and erythrocyte proportions were significantly lower (p<0.001) in cardioembolic thrombi than in large artery atherosclerosis thrombi. The composition of thrombi from undetermined aetiology strokes resembled that from cardioembolic strokes. CD3+ T cell and NET proportions were not significantly different between stroke subtypes. CD3+ density per unit area was associated with the occlusive site, being significantly higher in the anterior circulation than the posterior circulation (p=0.004). Cardioembolic strokes were more common in the anterior circulation than large artery atherosclerosis strokes (p=0.002). Recanalisation time was significantly longer for large artery atherosclerosis emboli than for cardioembolic emboli (p=0.032).
Conclusion There is significant heterogeneity in thrombus composition among different stroke subtypes. The quantitative assessment of thrombus composition may be a useful biomarker of stroke aetiology, and strokes of undetermined aetiology may be more likely to have a cardioembolic origin.
WHAT IS ALREADY KNOWN ON THIS TOPIC
There have been several histopathological studies of intracranial thrombi to establish associations between clot composition and clinicopathological outcomes, but the data are conflicting.
WHAT THIS STUDY ADDS
This is an article examining the relationship between thrombus composition and other clinical parameters and different aetiologies in patients with thrombus extraction. Our finding of significant heterogeneity in thrombus composition among different stroke subtypes marking stroke aetiology suggests that the quantitative assessment of thrombus composition may be a useful biomarker of stroke aetiology, and strokes of undetermined aetiology may be more likely to have a cardioembolic origin.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings contribute to an improved understanding of thrombus composition and its impact on stroke aetiology and tailoring therapeutic interventions.
Introduction
Cerebrovascular diseases are a major cause of disability, morbidity and death worldwide,1 especially through infarction caused by large cerebral vessel occlusion (LVO). Fortunately, advances in mechanical thrombectomy have improved clinical outcomes for patients who had a stroke. Further investigation of the retrieved thrombi offered useful insights for personalising the diagnosis, treatment, prevention and prognostication of LVO ischaemic stroke.
Thrombi are composed of erythrocytes, leucocytes, F+P (fibrin+platelets), neutrophil extracellular traps (NETs) and other clotting factors.2 In general, thrombi are heterogeneous, and their composition varies according to stroke subtype.3 It has been hypothesised that the quantitative analysis of retrieved blood clots could help determine the pathoetiology of stroke.4 5 Recently, the Clot Summit Group released a consensus statement based on contemporary research on clots and thrombi associated with strokes and reiterated their cellular and molecular heterogeneity, underscoring the potential significance of distinct thrombus components and compositions in stroke pathoetiology and providing a conceptual framework for subsequent research.6
Many histopathological studies of intracranial thrombi have established associations between clot composition and clinicopathological outcomes including stroke aetiology, imaging interventions, reperfusion therapy and prognosis.7 8 Nevertheless, the data are conflicting, in part due to challenges associated with acquiring thrombus specimens, limited sample sizes and insufficient data on the association between thrombus composition and aetiology. We, therefore, prospectively collected intracranial thrombus specimens from 132 patients with acute ischaemic stroke (AIS) undergoing endovascular therapy. Using H&E staining and immunohistochemical techniques, we systematically quantified clot composition with respect to the abundance of erythrocytes, leucocytes, F+P, CD3 and NETs. Our aim was to define robust associations between thrombus heterogeneity and stroke aetiology.
Methods
Patients and acquired thrombi
This was an analysis of consecutive thrombi obtained from patients diagnosed with AIS due to LVO treated at the third Xiangya Hospital, between January 2020 and September 2023. This study was a single-centre, a retrospective cohort study, involving patients with AIS-LVO, who underwent mechanical thrombectomy. In our study, thrombi were collected and then analysed using pathological staining techniques. Subsequently, we conducted a statistical analysis to examine the thrombi composition in relation to various clinical parameters, which allowed for a detailed evaluation of the thrombi characteristics and their clinical correlations.
The inclusion criteria were (1) confirmation of occlusion of the cervical carotid artery or intracranial internal carotid artery terminus, middle cerebral artery M1 segment or basilar artery through imaging and (2) Alberta stroke programme early CT score (ASPECTS) ≥6, posterior circulation ASPECTS ≥6 or meeting DAWN or DEFUSE-3 trial criteria.9 Exclusion criteria were patients with fragmented thrombi, thrombi of insufficient size for histopathological examination, or incomplete clinical data acquisition, and a diagnosis of Trial of ORG 10172 in Acute Stroke Treatment (TOAST-3) (small-vessel disease) or TOAST-4 (other identified causes). Comprehensive baseline clinicodemographic information such as sex, age and stroke risk factors was also collected.
Aetiological classification
Using the TOAST classification,3 we employed MRI, digital subtraction angiography, carotid artery colour Doppler ultrasonography, extended electrocardiography and screening for patent foramen ovale to ascertain the most probable stroke aetiology. Large-artery atherosclerosis (LAA) was defined as catheter angiography findings showing >50% stenosis or occlusion of the ipsilateral extracranial or intracranial carotid artery proximal to the occlusion site without evidence of potential sources of cardioembolism in other diagnostic studies. Cardioembolism was defined as at least one cardiac source for an embolus following a complete cardiologic workup including Holter monitoring (24 hours, period of hospitalisation after illness inset) and transthoracic echocardiography, as well as in the absence of imaging characteristics of LAA. Stroke of undetermined aetiology (SUE) was defined when no reliable aetiology or at least two aetiologies were found after a complete clinical, laboratory and imaging workup (eg, atrial fibrillation and permeable foramen ovale). Aetiology was classified as TOAST-1 (LAA), TOAST-2 (cardioembolism (CE)), TOAST-5 (SUE) based on established criteria and consensus guidelines.3 TOAST-3 (small vessel disease) and TOAST-4 (other determined causes) were excluded.
Mechanical thrombectomy
Endovascular procedures were performed by an experienced neurointerventionalist under general anaesthesia. Patients were treated with mechanical thrombectomy using either a stent retriever (Trevo ProVue Stentriever) or contact aspiration (Catalyst 6) as first-line treatment. According to the modified thrombolysis in cerebral infarction (mTICI) scale,10 successful recanalisation was defined as an mTICI grade of 2b or 3.
Clinical outcomes and prognosis
Patients were comprehensively assessed for postinterventional bleeding events according to European Cooperative Acute Stroke Study II criteria.11 A comprehensive neurological examination was also performed using the National Institute of Health Stroke Scale(NIHSS) score to assess neurologic status on admission, preinterventional and postinterventional thrombectomy, and at discharge. Functional outcomes were assessed according to the modified Rankin scale (mRS) score at 90 days.
Histopathology
Extracted thrombus specimens were immediately fixed in 10% phosphate-buffered formalin (Servicebio, Hubei, China), dehydrated and embedded longitudinally in paraffin. Three μm thick sections were cut and stained with H&E to observe the main thrombus components including red blood cells (RBCs), white blood cells (WBCs) and F+P. Additionally, immunohistochemistry was performed with antibodies targeting cluster of differentiation 3 (CD3) and citrullinated histone H3 (H3Cit, for NETs). Antigen retrieval was performed with EDTA buffer (Servicebio) in a microwave oven. The primary antibodies (CD3, ab16669,1:200;H3Cit,ab5103,1:200; all from Abcam, Cambridge, UK) were incubated overnight at 4°C, followed by incubation with the secondary antibody (goat anti-rabbit IgG,G23303,1:200;Servicebio) at 25°C. Positive staining was visualised using 3'-diaminobenzidine. Negative controls for immunohistochemistry were obtained by omitting the primary antibody.
Quantification of thrombus histology
In pathological-stained sections, the major thrombus components were quantified using semiautomated colour segmentation-based software (Orbit Image Analysis Software;www.orbit.bio),12 which calculates the percentage of each thrombus component (RBCs, WBCs, F+P and NETs) relative to the total thrombus area and counts the cells of the total thrombus area (immunohistochemistry-positive cells, CD3). Thrombus size was estimated according to the total number of pixels per specimen. To reduce bias from intrathrombus heterogeneity, we analysed the section of the largest longitudinal axis of the thrombus to represent the overall thrombus composition.
Statistical analysis
Continuous variables are presented as means and SD or medians and IQRs, depending on whether they follow a normal distribution. While categorical variables are presented as frequencies and percentages and examined using χ2 tests or Fisher’s exact tests. For the comparison of thrombus components between different stroke aetiology subtypes, one-way analysis of variance was used for RBC and F+P ratio following normal distributions, while the Kruskal-Wallis test was used for WBC, CD3 and NETs not following a normal distribution, Bonferroni multiple comparisons were performed. Independent sample t-tests or Mann-Whitney U tests were used to compare continuous variables between the two groups. Binary logistic regression analysis of thrombus components was performed to identify potential predictors of stroke pathogenesis. The receiver operator characteristic (ROC) curve was further used to identify biomarkers of most diagnostic value. ROC curve analysis established a cut-off value that determined whether any of the thrombotic components including RBCs, WBCs, F+P, CD3 and NETs could be used to differentiate stroke aetiology. All statistical analyses were carried out using SPSS software (IBM SPSS Statistics V.27), with statistical significance set at p<0.05. All figures were drawn using GraphPad Prism V.10.
Results
Baseline characteristics
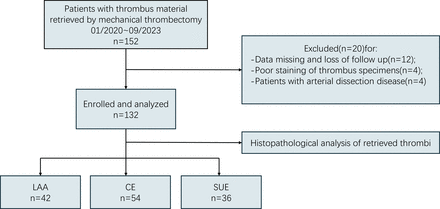
The clinical, interventional and histological characteristics are shown in table 1, which also shows clinical variables categorised by stroke aetiology. The initial study included 152 patients. After screening, 12 patients were excluded due to missing data or loss to follow-up, 4 were excluded because of poor staining of the thrombus specimens, and 4 patients with dissection were also excluded, as shown in figure 1. Finally, a total of 132 patients were included, with a mean age of 65.3±13.1 years, and 34.1% were female. According to TOAST classification criteria, 42 cases (31.8%) were classified as LAA, 54 cases (40.9%) were classified as CE and 36 cases (27.3%) were classified as SUE. 54 patients (40.9%) received tissue plasminogen activator, and there were no statistically significant differences in the proportion of RBCs, WBCs, F+P, NETs or CD3 in clots obtained from patients with and without tPA treatment(p=0.360, p=0.206, p=0.601, p=0.275 and p=0.563, respectively) (online supplemental figure 1). Successful reperfusion (TICI grade 2b or 3) was achieved in 121 (91.7%) of 132 patients, with a median number of manoeuvres of 2 (range 1–2). The median time from femoral artery puncture to successful reperfusion was 60 (range 40–87.75) min. No statistically significant differences in first-pass effect were observed among patients with varying stroke aetiologies (p=0.693). Furthermore, patients were classified into non-FPE and FPE groups, and a comparative analysis of the baseline demographic, clinical and procedural characteristics of these groups is provided in online supplemental table 1.
Supplementary data
Flow diagram of patients screened, enrolled and analysed. CE, cardioembolic; LAA, large artery atherosclerosis; SUE, stroke of undetermined aetiology;NIHSS, National Institute of Health Stroke Scale .
Clinical characteristics of the study population
Histopathological analysis
The 132 thrombi exhibited significant compositional heterogeneity. Among these constituents, F+P constituted the highest proportion, followed by RBCs, with WBCs the least abundant. Specifically, the mean F+P content was 51.46%, the mean RBC content was 40.97% and the median WBC content was 6.88%. The median CD3+ T cell content within the thrombus was 20.64 cells/mm², and the median NETs content was 1.03% Cholesterol crystals or calcified material were not identified in extracted clots. For further details, refer to haematoxylin staining, NETs staining and CD3 staining in online supplemental figures 2–4.
Thrombus composition varies by aetiology, the details are shown in table 2. The CE thrombi exhibited a significantly higher proportion of F+P compared with LAA thrombi (55.37%±15.19% vs 39.95%±19.37%, p<0.001) and a lower proportion of RBCs (38.21%±15.41% vs 51.19%±21.12%, p<0.001). Conversely, the F+P content of SUE thrombi was similar to that of CE thrombi (59.04%±18.30% vs 55.37%±15.19%, p=0.330) but significantly higher than LAA thrombi (59.04%±8.30% vs 39.95%±19.37%, p<0.001). Similarly, the RBC content in SUE thrombi was comparable to that of CE thrombi (33.19%±18.22% vs 38.21%±15.41%, p=0.201) but significantly lower than LAA thrombi (33.19%±18.22% vs 51.19%±21.12%, p<0.001). In our study, there were no statistically significant differences in the WBC composition among LAA, CE and SUE thrombi (7.61% vs 5.86% vs 8.18%, p=0.099). Similarly, there were no statistically significant differences in the CD3 (19.39% vs 20.64% vs 25.17%, p=0.545) and NETs (0.86% vs 1.07% vs 1.16%, p=0.509) content among LAA, CE and SUE thrombi (table 2).
Differences between thrombus composition and associated clinical data in stroke of different aetiologies
ROC curve
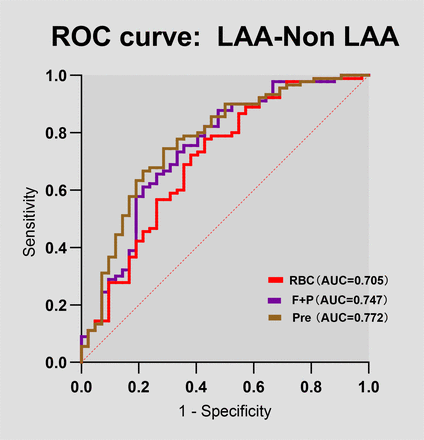
In a binary logistic regression analysis of thrombus composition, the percentage of RBC and F+P in thrombi was significantly associated with LAA and non-LAA (non-LAA stroke) thrombi. RBC and F+P composition was determined to be the most probable predictor of LAA stroke by ROC curves (RBC area under the curve 0.705 (95% CI 0.605 to 0.805), p=0.0002; F+P area under the curve 0.747 (95% CI 0.652 to 0.841), p<0.0001) (figure 2). Furthermore, the predictive probabilities derived from the two metrics, RBC and F+P, were computed through the construction of a logistic regression model. These probabilities were subsequently employed as the sole analytical metrics for the construction of the ROC curve (Pre, the joint prediction probability, area under the curve 0.772 (95% CI 0.683 to 0.862), p<0.0001) (figure 2), which had a moderate diagnostic value, indicating that the diagnostic model was effective.
Predictive value of thrombus components composition in discriminating stroke aetiology. The ROC curve displaying the performance of thrombus RBC, F+P content in discriminating a large artery atherosclerosis (LAA) aetiology from a non-LAA (non-LAA) aetiology. AUC, area under the curve; F+P, fibrin and platelet; Pre, the joint prediction probability; RBC, red blood cell; ROC, receiver operator characteristic; WBC, white blood cell.
Occlusion site
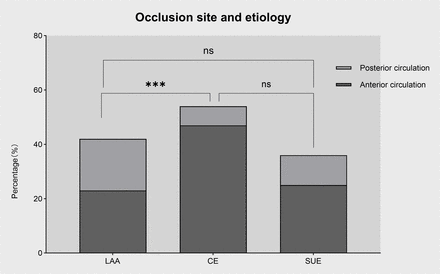
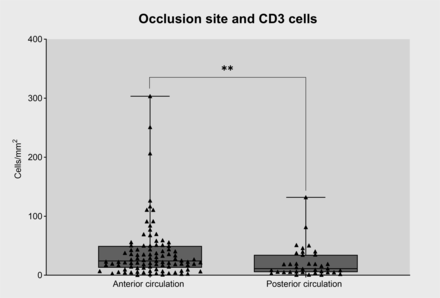
The most common site of occlusion was the internal carotid to middle cerebral artery(72%), while the vertebral-basilar artery was occluded in 28% of cases. Occlusions in patients with CE stroke (vs LAA stroke) were mainly in the anterior circulation (87% vs 54.8%, p=0.002) (figure 3). There were no significant differences in thrombus composition (erythrocytes, leucocytes, F+P and NETs) according to site of occlusion (p>0.05) (online supplemental figure 5). However, the proportion of CD3+ T cells was higher in thrombi in the anterior circulation compared with the posterior circulation (24.26% vs 11.07%, p=0.004) (figure 4).
Relationship between occlusion site and different aetiologies. There was a significant difference in occlusion site according to stroke subtype. Compared with LAA strokes, cardioembolic strokes were more likely to occur in the anterior circulation. ***p<0.001. CE, cardioembolic stroke; LAA, large artery atherosclerosis; SUE, stroke of undetermined aetiology.
Relationship between occlusion site and CD3+ cell content in intracranial thrombi. CD3+ cells were significantly more numerous in anterior circulation clots. **p<0.01.
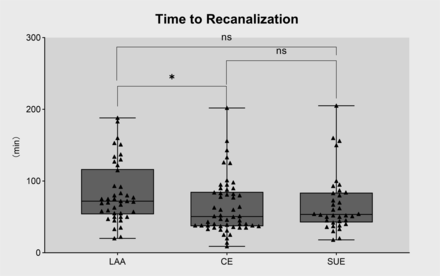
Puncture-to-recanalisation time
Reperfusion times were longer in patients experiencing LAA strokes than those with CE strokes (72 vs 50.5 min, p=0.032) (figure 5).
Association between recanalisation time and thrombus composition in patients with different aetiologies. Patients with LAA strokes experienced longer reperfusion times than those with cardioembolic strokes. *p<0.05 . CE, cardioembolic stroke; LAA, large artery atherosclerosis; SUE, stroke of undetermined aetiology.
Discussion
Our comprehensive quantitative histopathological analysis of emboli retrieved from LVO stroke patients revealed significant differences in composition between CE and LAA thrombi. Specifically, CE thrombi contained a higher proportion of F+P and a lower proportion of erythrocytes. While not statistically significant, SUE and CE thrombi were highly similar. We also detected a significant association between erythrocyte and F+P composition and specific aetiologies. Employing ROC analysis based on the main thrombus constituents, especially RBC predominant in LAA thrombi, we identified a probable predictive biomarker with a high AUC to differentiate LAA from non-LAA emboli. This finding facilitates the differentiation between LAA and Non-LAA emboli through quantitative histopathological analysis, thereby providing a basis for guiding future patient management strategies.
The rapid development of neurointerventional techniques has facilitated the study of thrombus morphology and histopathologic features. Despite new evidence, the link between thrombus histopathology and stroke aetiology and outcome remains unclear. Several previous studies have analysed the composition of thrombi removed by intervention in patients with LVO of different aetiologies, but the results have been variable and inconclusive. Some early studies indicated a high erythrocyte component in CE thrombi,13 14 whereas others reported a larger erythrocyte component in noncardiogenic or LAA thrombi,15–17 and there are also studies that have not found a difference in erythrocyte content of thrombus components between different aetiologies.18 A 2022 study19 concluded that erythrocyte content was higher in non-cardiac embolic aetiology and large atherosclerotic strokes compared with cardiac embolic aetiology, and fibrin content showed a significant association between cardiac embolic stroke and cryptogenic stroke, a correlation that is consistent with most of the literature, and with which our study is in agreement. Though a few studies have described the opposite, with Kim et al13 and Shin et al.20 Besides, the similarity in composition between cryptogenic and cardioembolic strokes suggests that many cryptogenic strokes may be reclassified as cardioembolic, which is similar to previous findings by Novotny et al.21 Additionally, our results may support a hypothesis that SUE mainly originate from CE emboli.
We also aimed to identify other biomarkers of stroke aetiologies by using immunohistochemistry to evaluate CD3+T cells and NETs in the thrombi. Previous studies have examined relationships between leucocyte composition in thrombi and aetiology, but the data have been limited and inconclusive. A recent study revealed an association between high leucocyte numbers in thrombi and a CE origin, but the analysis considered the entire WBC population without breakdown into specific subtypes.22 Although Dargazanli et al23 detected significantly more CD3+T cells in atherosclerosis-derived thrombi, their sample size was too small to be definitive. In addition, they chose to analyse the thrombus specimens in proximal-mid-distal sections, and, after observing all the sections, only those with the highest number of CD3+ cells were quantitatively analysed. This is not quite the same as our method, and, considering the possibility of intrathrombotic heterogeneity, we used the largest section of the longitudinal axis of the thrombus selected to represent the overall thrombus composition. While we found no significant differences in CD3+T cell composition between aetiological subgroups, consistent with Sporns et al,24 Berndt et al25 reported that anterior and posterior circulatory occlusion thrombi have different compositions, but they did not study the CD3+T cell component. Understanding these compositional differences could provide insights into future thrombus extraction strategies.
We also find that the number of CD3+T cells in anterior circulation thrombus was significantly higher than in posterior circulation thrombus, which is the first description of an association between CD3+T cell content and occlusion location. Many studies have indicated a notable reduction in the CD3+ cell population in patients with diabetes compared with their non-diabetic counterparts, implying a diminished presence of T cells engaged in immune responses within the diabetic cohort.26 27 Posterior circulation occlusive strokes are more likely to be associated with diabetes mellitus and metabolic syndrome compared with anterior circulation strokes,28 potentially explaining the observed lower CD3+T cell content within thrombi occluding the posterior circulation than those affecting the anterior circulation. This phenomenon might offer an additional rationale for the observed relationship between the proportion of CD3+T cells in thrombi and their location.
There are some data on the relationship between NETs, crucial contributors to thrombus formation and stroke aetiology. Laridan et al29 have found that neutrophils and NETs form important constituents of cerebral thrombi, they confirmed the specific presence of NETs in thrombi using colocalisation of H3Cit with extracellular DNA released by neutrophils, and the results of the study showed that the H3Cit positive area was 13.45% of the total thrombus area, and that cardiogenic thrombi had a higher level of H3Cit compared with other aetiologies. In our study, out of 132 thrombus specimens, NETs were not detected in five thrombus specimens, while NETs were present in the remaining thrombus specimens, with a positive area of up to 29.76%, which is similar to the results of the study by Laridan et al. However, we found no significant differences in NETs according to aetiology, consistent with Ducroux et al.30 However, unlike Genchi et al31 and Jabrah et al,32 the later study analysed 300 thrombus specimens and found that cardiogenic thrombus showed higher expression of neutrophils and NETs compared with atherosclerotic formation and cryptogenic thrombus, the diagnostic value of NETs as a biomarker needs to be further validated in a larger cohort.
Thrombi are not cleared in up to 20% of patients undergoing mechanical thrombectomy, and thrombus composition is a pivotal factor contributing to procedure failure.33 Unlike previous studies,7 34 Our analysis of associations between stroke aetiology and reperfusion time revealed that LAA strokes generally exhibit longer reperfusion times than CE strokes and is typically associated with RBC-rich clots, which we speculate might be related to the contribution of erythrocytes to thrombosis.35 In an environment of oxidative stress and mediated by proinflammatory factors, reactive oxygen species can increase the adhesion of erythrocytes to the vascular wall.36 This may even result in frequent and recurrent vascular occlusion crises.37 In addition to this, erythrocyte stagnation resulting from the interaction of erythrocytes with factor XIIIa increases thrombus size,38 and a longer thrombus length might be expected to generate increased friction and adhesion, in turn prolonging the recanalisation time for endovascular therapy. Second, structurally, erythrocyte-rich thrombi are usually surrounded by a dense platelet-rich shell, which is associated with thrombolytic resistance and also affects procedure duration to some extent.39 40 In addition, the occluded vessels in LAA stroke patients tend to have in situ stenosis, requiring a combined suction and stenting approach, which extends the procedure time, and remedial measures such as stent implantation or balloon dilatation are more often required after endovascular treatment, also possibly increasing the procedure time in LAA patients.
We explored potential associations between individual thrombus components and stroke aetiology using binary logistic regression analysis, with the accuracy of thrombus components in diagnosing stroke aetiology further assessed by ROC curve analysis. The results showed that RBC/F+P had high diagnostic value in predicting stroke aetiology with an AUC of 0.705/0.747. Thus, RBC/F+P can be used as a possible biomarker for predicting LAA stroke and stroke of other aetiologies. Therefore, in cases where the cause of stroke cannot be determined by conventional testing, pathological analysis of thrombus may be useful for providing clinical diagnostic clues that can contribute to a more accurate determination of stroke aetiology, guiding a more precise therapeutic regimen. The accurate identification of stroke aetiology significantly impacts subsequent clinical decision-making. For LAA stroke and cryptogenic strokes, antiplatelet therapy remains the treatment of choice, but, if potentially from CE sources, anticoagulation therapy might be warranted. Secondary prevention regimens remain controversial between aetiologies, future management strategies could consider the use of anticoagulants for cryptogenic stroke patients. Nevertheless, our data highlight how the histological features of acute thrombi provide insights into the mechanisms of thrombus formation and could form the basis of personalising secondary prevention strategies for patients who had a stroke.
Our study has several limitations. First, only thrombi retrieved through successful thrombectomy procedures were available for analysis, thus excluding thrombi from patients with failed reperfusion. Additionally, the use of intravenous r-tPA in some cases might have influenced the thrombus specimens, although we detected no difference in composition. Furthermore, manual segmentation of thrombus components by operators might have introduced selection bias. Moreover, mechanical thrombectomy may have misplaced or disrupted the original thrombus constituents, potentially impacting our histological analysis. The single-centre sample is also a limitation in our study, which may lack sufficient diversity and generalisability. H&E staining does not differentiate between platelets and fibrin, which might limit the interpretability of the data but not the primary conclusions. In our initial study, leucocyte quantification was conducted using H&E staining; however, this method may introduce inaccuracies in the quantitative analysis of leucocyte populations. In subsequent research, we intend to employ immunohistochemical staining techniques to enhance the specificity of leucocyte identification. This approach will allow for precise quantification of neutrophils and other leucocyte subtypes. By doing so, we aim to deepen our understanding of the relationships between various leucocyte subtypes and their ratios, in correlation with pertinent clinical parameters. Such investigations are anticipated to yield novel insights that could be significant for clinical applications. Additionally, this study did not include an analysis of NETs, T cells or other clot components in peripheral blood, preventing us from drawing any conclusions regarding potential correlations between blood and clot levels. However, we consider this an important avenue for future research. Finally, despite statistical analyses in large patient groups, this study still cannot differentiate individual patients belonging to the LAA, CE or SUE groups based on thrombus analysis.
Conclusions
We detected significant heterogeneity in thrombus composition between different subtypes of stroke, with LAA strokes characterised by a higher proportion of RBCs and cardioembolic strokes predominantly exhibiting high F+P ratios. Thrombus composition in SUE closely resembles that of cardioembolic stroke but significantly differs from LAA stroke, paving the way for distinguishing stroke aetiologies through quantitative histopathology.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Ethics Committee, Xiangya Third Hospital, Central South University, ChinaID:快 24071. Participants gave informed consent to participate in the study before taking part.
Footnotes
ZJ and JH contributed equally.
Contributors ZJ and JH designed the study. ZJ, JH, SH, RX, LR, YC, DX, PL, XL and YY collected and abstracted the data. SH, YY and XL contributed to the interpretation of data and critical revision of the manuscript. ZJ and JH wrote the first draft of the article, to which the other authors added comments. YY is the guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.