Abstract
Objective Limited evidence is available regarding the risk-benefit ratio of thrombolytic therapy in patients with stroke and renal impairment complications, particularly for the drug tenecteplase. Therefore, we examined the association of impaired renal function with the safety and efficacy of intravenous thrombolytic treatment (IVT) in patients with acute ischaemic stroke (AIS).
Methods A post hoc analysis of a randomised controlled trial (ClinicalTrials gov. NCT04797013) was conducted. Participants who received IVT with tenecteplase and alteplase (0.25 and 0.9 mg/kg, respectively) within 4.5 hours of symptoms onset were categorised based on their estimated glomerular filtration rate as follows: (1) ≥90 mL/min/1.73 m2,normal renal function; (2) 60–89 mL/min/1.73 m2, mildly decreased renal function; and (3) <60 mL/min/1.73 m2, moderately to severely decreased renal function. Patients stratified based on the normal renal function were used as the references. The primary efficacy and safety outcome were the percentage of patients achieving a modified Rankin Scale score of 0–1 at 90 days and the symptomatic intracranial haemorrhage (sICH) occurrence within 36 hours, respectively.
Results In intravenous tenecteplase-treated patients, mildly decreased renal function (OR 3.10; 95% CI: 1.41 to 6.78) and moderately to severely decreased renal function (OR: 8.03; 95% CI: 2.76 to 23.38) showed an association with a higher risk of all-cause mortality but not with sICH incidence compared with normal renal function. Among patients administered intravenous alteplase, those with a moderate-to-severe decrease in renal function exhibited an elevated risk of sICH (adjusted OR: 10.01; 95% CI: 1.61 to 62.15) and all-cause mortality (adjusted OR: 4.54; 95% CI: 1.48 to 13.91). Comparative treatment effects between tenecteplase and alteplase according to renal function grades showed no heterogeneity.
Conclusions A significant correlation was noted between kidney dysfunction and unfavourable outcomes in individuals with AIS who received treatment with either tenecteplase or alteplase.
WHAT IS ALREADY KNOWN ON THIS TOPIC
The influence of kidney impairment on clinical outcomes after stroke thrombolysis with alteplase remains debatable, while efficacy and safety profiles of tenecteplase in these patients are lacking.
WHAT THIS STUDY ADDS
Decreased renal function did not influence the efficacy outcomes, but was correlated to a high risk of all-cause mortality/symptomatic intracranial haemorrhage events in patients receiving tenecteplase and alteplase compared with that in individuals showing normal renal function. There was no heterogeneity in the comparative treatment effects between the two thrombolytic agents according to renal function grades.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Renal impairment should be considered when evaluating the risks and benefits of intravenous thrombolytic treatment with alteplase and tenecteplase.
Introduction
Approximately 20–35% of individuals experiencing an acute ischaemic stroke (AIS) have complications of chronic kidney disease (CKD),1 a factor that demands careful clinical consideration due to the complexities involved in managing their condition. Emergency intravenous thrombolytic treatment (IVT) is presently the preferred treatment for AIS, and alteplase is the recommended standard drug for individuals meeting the eligibility criteria according to the guidelines.2 Tenecteplase, a genetically modified version of tissue plasminogen activator, features an extended half-life and higher fibrin specificity than those of alteplase.3 These characteristics make it a beneficial thrombolytic agent, promoting quicker and more complete clot dissolution while reducing the risk of bleeding complications. Accumulating clinical trial data support that tenecteplase is equally effective as alteplase in treating AIS.4
The most severe complication of systemic thrombolysis is symptomatic intracranial haemorrhage (sICH).5–7 Renal function affects IVT efficacy in patients with AIS through intricate biological mechanisms, including disruptions in coagulation and platelet function, microvascular damage,8 alterations in haemodynamics,9 inflammatory responses, oxidative stress and endothelial impairment.10 11 The interplay among these mechanisms may significantly affect both the efficacy and safety of thrombolytic therapy.6 12 This raises the question of whether renal dysfunction is a significant issue in thrombolytic therapy. Current studies present conflicting evidence on the association of renal impairment and post-thrombolytic sICH risk. Furthermore, there is a paucity of data on how reduced renal function affects the outcomes for patients treated with tenecteplase following AIS.
Characterisation of the risks associated with varying levels of renal function will facilitate the development of appropriate screening and prevention strategies. In this context, we report a post hoc study of TRACE-2 (tenecteplase vs alteplase in acute ischaemic cerebrovascular events) trial13 to evaluate how reduction in the renal function of patients with AIS affects the safety and efficacy profiles of tenecteplase and alteplase used for IVT in patients with AIS.
Methods
Patient population
A post hoc study of the TRACE-2 trial13 was conducted. Study design and statistical analysis plan were reported previously.13 14 In brief, the TRACE-2 trial was a randomised, prospective, multicentre, blinded-endpoint, open-label, controlled phase III trial with an aim to test non-inferiority. We enrolled 1430 patients in the trial; enrolment was completed within a period of 4.5 hours after stroke onset. Participants were randomly allocated to receive either tenecteplase (up to 25 mg at the dose of 0.25 mg/kg) or alteplase (up to 90 mg at the dose of 0.9 mg/kg) at a 1:1 ratio. The TRACE-2 trial was registered at ClinicalTrials.gov (NCT04797013).
Outcomes
The primary effectiveness outcome was the proportion of patients showing a modified Rankin Scale (mRS) score of 0–1 at 90 days. The following secondary efficacy outcomes were considered: mRS score of 0–2 at 90 days; significant improvement in neurological status on the National Institutes of Health Stroke Scale (NIHSS), determined as attenuation of ≥4 points and a score of ≤1 at 24 hours and 7 days, or on discharge from the hospital; European Health-Related Quality of Life (EHRQOL) at 90 days and the percentage of individuals showing a Barthel index score of ≥95 at 90 days.
The primary safety outcome evaluated was the sICH incidence within 36 hours, as defined by the European Cooperative Acute Stroke Study III.15 Additional safety outcomes investigated were parenchymal haematoma type 2 (PH2)16; any form of intracranial haemorrhage as defined by the GUSTO criteria17 and mortality from any causes within a timeframe of 90 days from disease onset. Severe adverse events (SAEs) and adverse events (AEs) were recorded within a period of 90 days. The characterisation of AEs and SAEs was defined based on the guidelines set by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice E6 (R2).
Renal impairment definition
Estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation.18 Participants were grouped into three eGFR categories: (1) moderately to severely declined renal function, <60 mL/min/1.73 m2; (2) mildly declined renal function, 60–89 mL/min/1.73 m2; and (3) normal renal function, ≥90 mL/min/1.73 m2.
Statistical analysis
We analysed the influence of reduced renal function on the outcomes of patients with AIS who underwent IVT; patients with normal renal function were considered the reference group. We calculated the ORs and 95% CIs through binary logistic regression models for estimating the correlations between eGFR as a categorical variable and the efficacy and safety outcome. A general linear model was used to calculate β-coefficient with 95% CI for the outcome of EHRQOL based on the visual analogue scale. The multivariate analysis models were adjusted for potential confounders that may clinically affect the outcomes, including age, sex, baseline NIHSS score, history of hypertension, prior use of antiplatelet and anticoagulant agents, onset-to-needle time and bridging thrombectomy.19 20 Restricted cubic spline models with 4 knots at 30, 60, 90 and 120 mL/min/1.73 m2 of eGFR were used.
Additionally, we compared the differences in safety and efficacy outcomes between the alteplase and tenecteplase treatment groups; the alteplase group was used as a reference group, with stratification based on the eGFR category. Ordinal logistic regression was used to compare the differences in ordinal 90-day mRS scores between the treatment groups, and Cochran-Mantel-Haenszel χ² test was used to compare the other efficacy and safety outcomes by considering the site effect. The interaction p values between the eGFR groups and treatment allocation were further calculated. To confirm whether the findings were reliable, we conducted sensitivity analyses by excluding patients with a history of antiplatelet and anticoagulant medications before stroke onset. SAS 9.4 (SAS Institute Inc, Cary, North Carolina, USA) was used for all statistical analyses.
Results
Baseline characteristics
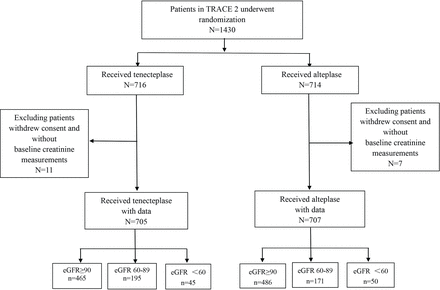
Of the 1430 eligible patients recruited in the TRACE-2 trial, 1412 (98.7%) with available baseline creatinine measurements and 90-day outcome data were analysed (figure 1, table 1). Among the enrolled participants, 706 patients each were allocated to receive either tenecteplase or alteplase treatment. Of the 1412 patients, 67.4%, 25.9% and 6.7% had normal, mild and moderately declined renal function, respectively.
Flow chart of the study. eGFR, estimated glomerular filtration rate; TRACE-2, tenecteplase vs alteplase in acute ischaemic cerebrovascular events.
Baseline characteristics of intravenous thrombolysis-treated patients according to renal function
Patients experiencing declined renal function tended to be older; had higher body mass index values; were more frequently female; had a greater incidence of hypertension, ischaemic heart disease and arrhythmia; and had prior usage of antiplatelets, lipid-lowering medications, anticoagulants and antihypertensives compared with those without declined renal function. No differences were observed in the NIHSS scale, mRS score before stroke, onset-to-needle time or bridging thrombectomy between the groups stratified by treatment and renal function.
Association of renal function impairment with outcomes in tenecteplase-treated patients
Table 2 presents the unadjusted and adjusted ORs comparing the associations of renal impairment with efficacy endpoints in AIS participants treated with tenecteplase. Reduced renal function was related to reduced probability of optimal functional outcomes (mRS 0–1 and 0–2) compared with normal renal function in the unadjusted model. However, in the multivariate analyses, no significant associations were found regarding functional outcomes within 90 days after adjusting for confounding risk factors.
Efficacy outcomes according to eGFR category in patients treated with tenecteplase
As shown in table 3, the multivariate comparison did not indicate any increase in the rates of sICH or PH2 within 90 days in patients with renal impairment. However, even after multivariable adjustment, mildly reduced renal function (OR: 3.10; 95% CI: 1.41 to 6.78) and moderately reduced renal function (OR: 8.03; 95% CI: 2.76 to 23.38) remained independently associated with all-cause death compared with normal renal function, and a tendency towards higher frequency of mortality with gradual increase in renal insufficiency was observed.
Safety outcomes according to eGFR category in patients treated with tenecteplase
Association of renal impairment with outcomes in alteplase-treated patients
Table 4 shows the univariate and multivariate ORs as efficacy outcome measures in the comparison of alteplase-treated patients with AIS at different degrees of renal impairment compared with those with preserved kidney function. Multivariate analyses revealed no significant differences in the proportion of patients with mRS scores 0–1 and 0–2 at 90 days across different stages of renal dysfunction.
Efficacy outcomes according to eGFR category in patients treated with alteplase
As shown in table 5, ORs of sICH incidence (adjusted OR: 3.20; 95% CI: 0.92 to 11.11) and all-cause death (adjusted OR: 0.93; 95% CI: 0.35 to 2.41) were not higher in patients with a mild reduction in renal function compared with patients with normal renal function. However, a moderate level of renal dysfunction was linked to a significantly greater risk of sICH within 36 hours (adjusted OR: 10.01; 95% CI: 1.61 to 62.15) and a 4.5-fold increase in all-cause mortality risk (adjusted OR: 4.54; 95% CI: 1.48 to 13.91) at 90 days.
Safety outcomes according to eGFR category in patients treated with alteplase
Comparison of safety and efficacy profiles of alteplase and tenecteplase in relation to renal function
The safety and efficacy profiles of alteplase and tenecteplase were compared in relation to renal function in patients with AIS. The results are provided in online supplemental tables S1–S3 and figure S1. In the mild CKD group, 58.8% versus 51.5% patients achieved the primary efficacy endpoint (mRS score 0–1) in the two groups, respectively (OR: 1.14: 95% CI: 0.95 to 1.37). In moderate-to- severe patients with CKD, the primary efficacy outcome was achieved in 46.7% of patients receiving tenecteplase and 52.0% of patients receiving alteplase (OR: 0.86; 95% CI: 0.59 to 1.27). No remarkable differences were noted in primary or secondary efficacy endpoints between the treatment groups across the different renal function categories (p for interaction=0.55, 0.79, 0.82 and 0.38, respectively). The primary safety outcome, that is, sICH within 36 hours, occurred in 2.9% and 3.6% of patients in the alteplase and tenecteplase treatment groups, respectively, with a mild decrease in renal function (OR: 1.41; 95% CI: 0.49 to 4.08) and in 6.0% and 2.2% (OR: 0.40; 95% CI: 0.04 to 4.30) of patients with moderately to severely declined renal function, respectively. eGFR did not exhibit significant interactions with the randomised treatment in safety outcomes (online supplemental table S3). Overall, the level of renal function did not alter the therapeutic effect of tenecteplase compared with that of alteplase.
Supplementary data
Association of renal impairment with outcomes in the overall cohort
The pooled adjusted ORs and 95% CI for mRS score 0–1, sICH and all-cause mortality for all randomised patients who received thrombolysis treatment are shown in online supplemental figure S2. There was a tendency for a n-shaped relationship between eGFR and ORs for the number of patients with mRS score of 0–1; ORs showed a steady increase at eGFR<60 mL/min/1.73 m2 and a significant decrease at eGFR >90 mL/min/1.73 m2. In contrast, restricted cubic splines showed a monotonically inverted curved association between eGFR and sICH and all-cause mortality risks, wherein ORs increased linearly with a decrease in eGFR. The sensitivity analysis results were in accordance with the main analysis, showing that renal function impairment was related to sICH and all-cause mortality but not with functional results (online supplemental tables S4–S5).
Discussion
This post hoc study of the TRACE-2 trial is the first report to describe the association of impaired renal dysfunction with outcomes in patients receiving intravenous tenecteplase for AIS. Compared with normal renal function, reduced renal function was found to be correlated with a higher risk of all-cause mortality but not with sICH events in the tenecteplase treatment group. However, in the alteplase group, reduced renal function was correlated with a higher risk for sICH and all-cause mortality. The effects of interactions between eGFR and treatment allocation on efficacy and safety endpoints were not significant, suggesting that renal function did not alter the comparative risk-benefit profiles of tenecteplase versus alteplase.
The kidney, being a highly perfused organ and serving as a blood filter, has a pivotal function in regulating haemodynamics and blood components, thereby directly influencing coagulation processes.21 First, as CKD progresses, patients begin to exhibit platelet dysfunction due to certain uremic toxins and altered function of platelet glycoproteins (eg, GPIIb/IIIa),22 which result in impaired platelet adhesion and aggregation. Second, renal failure may lead to an imbalance between clotting and anticoagulant factors,9 thereby affecting blood coagulability and increasing the risk of cerebral haemorrhage. Third, patients with CKD often present with hypertension and systemic inflammation, which can damage the vessel wall and increase the risk of cerebral haemorrhage. Fourth, poor kidney function may lead to endothelial dysfunction, affecting the elasticity and repair capacity of blood vessels.9
Compared with alteplase, tenecteplase exhibits a greater specificity to fibrin, high resistance to PAI-1 and extended plasma half-life.23 24 It also has a better safety profile.25 On systemic injection, the plasma half-life of tenecteplase was approximately 22 min; tenecteplase is mainly metabolised in the liver.26 Although CKD was found to be correlated with unfavourable outcomes in patients undergoing IVT, the present study showed that the safety and efficacy profiles of the two thrombolytic agents were not significantly different in patients with reduced renal function. Previous research using rat models of bilateral nephrectomy also showed no changes in the half-life or pharmacokinetics of alteplase.27 Both alteplase and tenecteplase are primarily metabolised in the liver rather than in the kidney,28 which may partly explain the absence of significant differences in the outcomes between the two treatments in patients showing reduced renal function. The combined results of the meta-analysis29 and randomised controlled trials (RCTs)30 bolster the evidence supporting that tenecteplase and alteplase exhibit comparable efficacy in the treatment of patients with AIS. Our post hoc study of the TRACE-2 trial is the first randomised comparison of the effects of alteplase and tenecteplase on stroke outcomes in this high-risk population. Overall, our results suggest that CKD does not modify the non-inferiority of tenecteplase to alteplase.
Recently, a meta-analysis of 60 486 patients with AIS receiving alteplase therapy revealed a correlation of CKD with an increased risk of ICH.6 Several cohort studies reported similar results.20 31 On the contrary, a retrospective study indicated that, in patients with ischaemic stroke who were administered intravenous alteplase, the presence of all stages of CKD led to increased, unadjusted likelihoods of sICH or severe systemic haemorrhage; however, these relationships were substantially attenuated after adjusting for baseline demographics and other risk factors. Similarly, a recent post hoc analysis from the ENCHANTED trial failed to identify independent associations between moderate-to-severe CKD and sICH.32 However, the criteria and definitions of bleeding, confounding risk factors adjusted in the multivariable model and formulas used for the estimation of GFR were different among these studies, which could partly explain the disparity in the results. A comparison of CKD versus normal renal function in patients with AIS undergoing IVT with tenecteplase is yet to be investigated. This study showed that, after multiple adjustments for confounding risk factors, there was no increase in sICH risk in tenecteplase-treated participants with reduced renal function.
Our results suggest that the all-cause mortality risk increased in patients with impaired renal function compared with that in patients with normal renal function after both thrombolytic treatments. However, the increased risk of mortality did not seem to be caused by an excess of sICH or a neuro-deficit. Renal insufficiency has been demonstrated to independently correlate with poor prognosis of stroke.33 However, the mechanisms underlying the high mortality rate associated with impaired renal function in IVT-treated patients remain unknown. The postulated explanations include the coexistence of renal dysfunction with other detrimental conditions, such as chronic inflammation, anaemia, oxidative stress and endothelial dysfunction,34 rather than the paradoxical effects on haemostasis typically associated with CKD.9 Previous studies have further argued that the increased mortality in patients with CKD may be related to indirect causes, such as an increased risk of pneumonia, sepsis and non-vascular events.20 32 35 A previous study indicated no heterogeneity in comparative treatment effects between 0.9 mg/kg and 0.6 mg/kg alteplase based on renal dysfunction grades.32 However, no studies have compared the safety and efficacy profiles of different tenecteplase doses in patients exhibiting moderate-to-severe renal insufficiency in the context of thrombolysis-treated AIS. Therefore, RCTs should be conducted to assess whether low-dose tenecteplase offers advantages over the standard dose for those experiencing both renal impairment and stroke.
Our study had some limitations. First, the number of patients showing moderate-to-severe renal impairment was comparatively low. However, our results suggest that even mild renal impairment correlated to higher risk of all-cause death after IVT with tenecteplase, providing evidence that impaired kidney function is a significant indicator of mortality risk. Second, the estimation of renal function was based on a single measurement of creatinine performed at the start of the study, which was determined in an acute hospital setting and may be impaired by the consequences of acute stroke. Nonetheless, we compared the renal function data at admission and 7 days after randomisation and found that the renal function in most of the enrolled patients was stable (data not shown). Third, it was challenging to establish definitive conclusions regarding the benefits and risks of IVT compared with controls not using IVT in the CKD population. Nonetheless, this research offers important insights into contemporary practices regarding the current practice of intravenous thrombolysis, especially with tenecteplase, in CKD-endemic areas, which may improve AIS management in the CKD population.
In summary, our study indicates that renal impairment did not affect the 90-day efficacy of IVT, but was associated with safety outcomes, including sICH within 36 hours and 90 days, as well as all-cause mortality within 90 days, in individuals with AIS. However, the efficacy and safety profiles showed no variations between the alteplase and tenecteplase treatment groups across the renal function categories. Our overall findings suggest that reduced renal function could be a potential risk factor affecting the prognosis of patients with AIS undergoing IVT treatment. Consequently, eGFR should be incorporated into the risk assessment score for evaluating the risk of sICH. Additional research is essential to clarify the significance of eGFR in the clinical screening process for IVT treatment in patients with early cerebral ischaemia.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and the principles of the Declaration of Helsinki. All participants gave informed consent before taking part. This study obtained ethics approval of Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University with number YW2020-046-04. The trial protocol received approval from the ethics committee and each participating site (IRB approval number: KY2019-035-02).
Footnotes
Contributors WD had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: YZ, YW and WD. Supplying patients: XM. Drafting of the manuscript: YW and YZ. Critical revision of the manuscript for important intellectual content: WD and YoW. Statistical analysis: YP and MW. Study supervision of the project: YoW, XM and YiW. Guarantors: WD and YZ.
Funding This study was funded by Key R&D Program of China (2017YFC1308204),This study was funded by Key R&D Program of China (2017YFC1308204),
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.