Abstract
Background We aimed to investigate the relationships between metabolic syndrome (MetS) and its severity score (Metsss) with asymptomatic intracranial arterial stenosis (aICAS) while also assessing the additional effect of high-sensitivity C reactive protein (hsCRP).
Methods This cross-sectional study included 2390 individuals who underwent health examinations at our centre from June 2019 to August 2023. Participants received physical examinations, laboratory tests and magnetic resonance angiography evaluations. MetS was defined by the modified acknowledged criteria and quantified by Metsss. Logistic regression, interaction analysis and mediation analysis were employed.
Results Among the 2390 participants, 135 (5.65%) had aICAS, and 726 (30.40%) had MetS. After adjusting for confounders, MetS was significantly associated with aICAS (OR: 1.68, 95% CI: 1.16 to 2.43, p=0.006). The prevalence of aICAS increased significantly from 3.6% to 8.6% as the number of MetS components increased. Higher quartiles of Metsss also significantly increased aICAS risk (P for trend <0.001). After multivariable adjustment, MetS (p=0.001) and elevated Metsss (p<0.001) were only associated with posterior circulation aICAS (vs anterior). Furthermore, participants with both MetS and elevated hsCRP levels had a greater risk for aICAS (OR: 2.32, 95% CI: 1.36 to 3.96, p=0.002). hsCRP mediated the association between MetS and alCAS in participants ≤65 years old.
Conclusions MetS and Metsss were independently associated with the risk of aICAS. The mediating effect of hsCRP on the relationship between MetS and aICAS appears to be age-dependent. These findings offer valuable insights into clinical decision making of aICAS and further improve the primary stroke prevention.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Metabolic syndrome (MetS) was associated with asymptomatic intracranial arterial stenosis (aICAS) using transcranial Doppler (TCD) or magnetic resonance angiography (MRA) when TCD detections were positive. MetS severity score was significant predictors of ICAS.
WHAT THIS STUDY ADDS
We used MRA to detect aICAS in neurologically healthy cohort, finding that both MetS and its severity score are excellent independent risk factors of aICAS. The combination of MetS and elevated levels of high-sensitivity C reactive protein (hsCRP) is linked to an increased risk of aICAS, with hsCRP serving as a significant mediator in the relationship between MetS and aICAS.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The application of this combined test in the clinical practice of aICAS may help clinical decision making and further improve the primary stroke prevention.
Introduction
Intracranial atherosclerosis stenosis (ICAS) is one of the leading causes of stroke worldwide. It is closely associated with high morbidity and mortality rates.1 2 Previous studies have predominantly focused on symptomatic ICAS.3
Asymptomatic ICAS (aICAS) is increasingly recognised as a common cause of cognitive impairment, dementia and silent cerebral infarctions.3 4 As the degree of stenosis worsens, the risk of vascular events increases correspondingly. Thus, proactive management of risk factors in patients with ICAS to prevent their first stroke may yield greater societal benefits compared with interventions after stroke occurrence.5
A collection of metabolic dysfunctions, such as central obesity, hypertension, dyslipidaemia and impaired glucose tolerance, define metabolic syndrome (MetS).6 It has been reported to be associated with the development of atherosclerotic cardiovascular disease (CVD), diabetes, chronic kidney disease7 and even an increased risk of cancer, all of which contribute to rising healthcare costs.8 Limited studies have explored the relationship between MetS and aICAS,9 10 mostly using transcranial Doppler (TCD) to diagnose arterial stenosis. To better reflect the severity of MetS, a continuous severity score (Metsss) has been developed. Beyond its clinical relevance to CVD, Metsss has been shown to predict ICAS and even future intracranial atherosclerotic strokes.11 However, the relationship between Metsss and aICAS remains inadequately explored.
Several studies suggest that high-sensitivity C reactive protein (hsCRP) significantly enhances the prediction of future cardiovascular events (CVEs) in middle-aged adults, particularly those under 65 years, independent of MetS.12 13Previous studies have indicated that hsCRP serves as a mediating factor in some atherosclerosis-related diseases, such as the relationship between the triglyceride-glucose index and major CVEs,14 and that between insulin resistance and prognosis of non-diabetic ischaemic stroke.15 However, the causal pathway between MetS, inflammation and risk of aICAS has not been investigated.
Herein, we aimed to investigate whether MetS and its severity score serve as predictors of the risk of aICAS and to analyse whether hsCRP exerts any additional effects in this relationship.
Methods
Population
This was an observational study. We selected eligible participants (n=3853) from the Health Management Centre of Beijing Tiantan Hospital, Capital Medical University, using health examination registration data collected between June 2019 and August 2023.
Assessments included questionnaires, physical examinations, laboratory tests and magnetic resonance angiography (MRA). Exclusion criteria were (1) a history of neurological diseases, such as ischaemic or haemorrhagic stroke, transient ischaemic attack or severe neurologic deficit (n=137); (2) incomplete baseline data (n=1279); and (3) baseline hsCRP levels ≥10 mg/L (n=47), as temporarily elevated inflammation levels may be associated with acute illness and could skew results.16 Detailed information is presented in a flowchart in online supplemental additional file 1: fig.S1.
Supplementary data
Ultimately, 2390 neurologically healthy participants were included in the analysis. This study adhered to the principles of the Declaration of Helsinki and was approved by the Central Ethics Committee of Beijing Tiantan Hospital. Written informed consent was obtained from all participants.
Definition of MetS and Metsss
MetS was defined according to the criteria established by the National Cholesterol Education Program Adult Treatment Panel III in 2005. Participants were considered to have MetS if they met three or more of the following criteria: (1) waist circumference ≥85 cm for women or ≥90 cm for men; (2) serum triglyceride (TG) levels ≥1.70 mmol/L; (3) serum high-density lipoprotein cholesterol (HDL-C) levels <1.3 mmol/L for women or <1.0 mmol/L for men; (4) fasting serum glucose (FBG) levels ≥5.6 mmol/L or use of antidiabetic drugs; and (5) blood pressure greater than 130/85 mmHg or use of antihypertensive drugs. Notably, we adjusted the waist circumference cut-off values to be specific for the Chinese population.17
The MetS severity score was calculated using a purpose-built R package, ‘pscore’, following the method described by Wiley and Carrington.18
Assessment of aICAS
Magnetic resonance angiography was performed using a 3T Siemens scanner (Skyra, Erlangen, Germany). Details of the MRI protocol, image analysis, quality control and reliability have been reported.19
The reconstructed time-of-flight MRA sequences were used to assess intracranial stenoses. Stenosis was classified according to the standard method used in the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) study, into five categories: no visible stenosis, <50%, 51%–70%, 71%–99% and occlusion.20 For this analysis, stenosis of ≥50% was diagnosed as aICAS.4
With a 0.852 kappa coefficient between their judgments, two skilled assessors independently assessed the images for vascular stenosis while masking the clinical information and the consultant neuroradiologist report. In cases of dispute, decisions were made by a third assessor. The intracranial arteries assessed included the supraclinoid and cavernous segments of the bilateral internal carotid arteries (ICA), anterior cerebral artery (ACA, A1–A3 segments), middle cerebral artery (MCA, M1–M4 segments), posterior cerebral artery (PCA, P1–P3 segments), intracranial segment of the vertebral artery (VA) and basilar artery (BA).4
Assessment of covariates
The detailed methods of baseline data collection, such as interviews, medical examinations and laboratory tests, have been previously reported.21 The definitions used for medical history of hypertension, diabetes, dyslipidaemia, coronary heart disease and smoking were also consistent with those used in previous studies.21 The levels of hsCRP were assayed on a Roche Modular P800 analyser (manufactured by Roche in Basel, Switzerland) using a turbidimetric immunoassay provided by Ji’en Technique Co. Ltd. (located in Shanghai, China). Both the intra-assay and interassay variation coefficients for hsCRP levels were determined to be 2.5% and 2.0%, respectively.
Statistical analysis
As all the continuous variables were skewed in distribution, they were expressed as medians (interquartile ranges, IQRs). Categorical variables are presented as frequencies (percentages). The participants were divided into three Body Mass Index (BMI) groups (<24.00 kg/m2, 24.00–27.99 kg/m2 and ≥28.00 kg/m2). According to the quartiles of hsCRP, monocyte-to-high-density lipoprotein-cholesterol ratio (MHR), neutrophil-lymphocyte ratio (NLR), homocysteine, low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), apolipoprotein A1 (ApoA1), apolipoprotein B (ApoB) and Metsss, each individual was split into four groups. Mann-Whitney and χ² tests were used to compare the MetS and non-MetS groups. Univariate and multivariate logistic regression were used to assess the associations of baseline markers, including MetS, with aICAS, expressed as ORs with 95% CIs. Demographic factors and predictors that significantly related to aICAS in univariate logistic regression analyses were potential confounding factors.
To clarify the association of MetS combined with hsCRP with aICAS, the subjects were categorised into four subgroups on the basis of baseline hsCRP levels and the presence of MetS (No MetS and 1st–3rd hsCRP quartile, MetS and 1st–3rd hsCRP quartile, No MetS and 4th hsCRP quartile, MetS and 4th hsCRP quartile). In addition, additive and multiplicative scales were used to analyse the interactions.22 Using three different metrics—relative excess risk owing to interaction (RERI), proportion attributable to interaction (AP) and Synergy Index (SI)—we assessed additive interactive effects. We also analysed multiplicative interactions with cross-product terms of MetS with hsCRP. The ‘interaction’ package from R was used for the calculations.
We used causal mediation analysis with the ‘mediation’ package in R to assess whether hsCRP (continuous) mediates the relationship between MetS (dichotomous) and aICAS (dichotomous).23 Logistic and linear regression models were used. To ensure stable estimates, a total of 1000 bootstrap resamples were applied.
Several previous prospective studies have shown that hsCRP provides significant additional predictive value beyond MetS for CVEs in middle-aged individuals (primarily those less than 65 years of age).24 Based on these findings, we conducted a stratified analysis by age to investigate the additive role of hsCRP in the association between MetS and aICAS among participants aged 65 years and younger. Additionally, interaction and mediation analyses were also explored within this subgroup.
All analyses were carried out with R version 4.1.1 (R Core Team, 2021). We considered p values less than 0.05 to indicate statistical significance. All tests were two-tailed.
Results
Subject characteristics
Among the 2390 eligible, neurologically healthy subjects in the final study sample, 1469 (61.46%) were men and their median age was 52.00 years (IQR, 44.00–58.00). Asymptomatic ICAS was detected in 135 (5.65%) participants, and 726 (30.40%) study participants had MetS. In participants with aICAS, the PCA was most commonly affected artery (45 cases), followed by the ICA (39), MCA (37), ACA (17), VA (11) and BA (2). Of these, 21 participants had aICAS affecting more than one artery. Approximately 80 cases involved the anterior circulation, 46 cases involved the posterior circulation and 9 cases involved both. The prevalence of stenosis at 51%–70%, 71%–99% and occlusion was 3.8% (90 cases), 1.1% (27) and 0.8% (18), respectively. The baseline characteristics according to the MetS classification are shown in table 1. Compared with the subjects without MetS, those with MetS tended to be older, male and smokers. Furthermore, they had higher levels of systolic blood pressure (SBP), diastolic blood pressure (DBP), BMI, waist circumference, ApoB, TG, FBG, hsCRP, NLR, MHR, homocysteine, uric acid and Metsss, as well as a higher incidence of hypertension, diabetes mellitus, coronary heart disease and dyslipidaemia (all p values <0.05). As expected, this group had relatively lower levels of HDL-C and ApoA1. No significant difference was observed with respect to TC or LDL-C. The subgroup aged ≤65 years consisted of 2202 subjects. Baseline characteristics, stratified by participants over or not greater than 65 years old, are presented in the online supplemental additional file 1: table.S1.
Baseline characteristics of the participants with and without MetS
Associations between MetS and aICAS
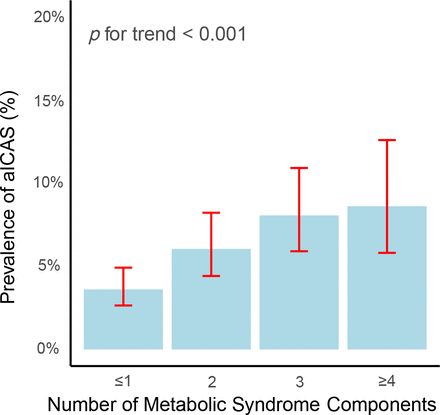
Table 2 presents the results of univariate logistic regression analyses examining the associations between baseline characteristics and aICAS. The following predictors were significantly related to aICAS: age, hypertension, diabetes mellitus, coronary heart disease, MHR, NLR and hsCRP (all p values <0.05). In the subgroup ≤65 years old, only MetS, hsCRP and coronary heart disease were significantly associated with aICAS (online supplemental additional file 1: table.S2). As presented in figure 1, the prevalence of aICAS increased significantly from 3.6% to 8.6% with an increasing number of MetS components.
Univariate logistic regression analysis for associations with aICAS
Prevalence (95% CI) of aICAS stratified by the number of metabolic syndrome components (unadjusted data). aICAS, asymptomatic intracranial arterial stenosis.
The associations among MetS, the number of MetS components and Metsss with aICAS are shown in table 3. MetS is significantly linked to aICAS (OR: 1.68, 95% CI: 1.16 to 2.43, p=0.006) after adjusting for potential confounders (including age, sex, coronary heart disease, NLR, MHR and hsCRP). Additionally, individuals with more MetS risk factors are more susceptible to aICAS. Compared with those with ≤1 risk factor, participants with 3 or ≥4 MetS risk components were significantly correlated with aICAS (≥4 vs ≤1: OR: 2.10, 95% CI: 1.20 to 3.70, p=0.010; 3 vs ≤1: OR: 1.97, 95% CI: 1.21 to 3.23, p=0.007; P for trend=0.002). Metsss is also an independent predictor of aICAS. Per SD increase in Metsss was linked with a 36% higher risk of aICAS (95% CI: 1.15 to 1.61, p<0.001). Compared with that of the Q1 group of Metsss, the risk of aICAS increased progressively from Q2 to Q4, with the Q4 group showing a significant association with aICAS (Q4 vs Q1: OR: 2.34, 95% CI: 1.32 to 4.13, p=0.004; P for trend <0.001). Therefore, metabolic syndrome, whether measured as a dichotomous criterion or a continuous severity score, is an independent predictor for aICAS. Furthermore, in the multivariate logistic regression analysis, MetS (OR: 2.84; 95% CI: 1.54 to 5.23; p=0.001) and elevated Metsss (OR: 2.93; 95% CI: 1.61 to 5.33; p<0.001) were solely correlated with posterior circulation aICAS (vs anterior) after adjusting for age, gender, coronary heart disease and hsCRP (online supplemental additional file 1: table.S3).
Multivariate logistic regression analysis for associations with aICAS
Joint associations of MetS and hsCRP with the risk of aICAS
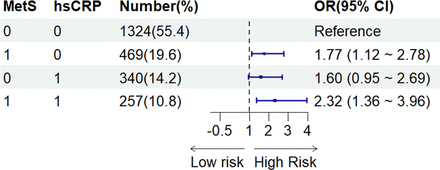
As illustrated in figure 2, in the entire cohort, taking those without MetS and with low levels of hsCRP (Q1–3) as reference, subjects who had both MetS and high hsCRP levels (Q4) were at significantly higher risk of aICAS (OR: 2.32, 95% CI: 1.36 to 3.96, p=0.002), and this OR was greater than that of subjects with only MetS (OR: 1.77, 95% CI 1.12 to 2.78, p=0.014) or high levels of hsCRP (OR: 1.60, 95% CI 0.95 to 2.69, p=0.076) (adjusting for all confounders). Similar results were observed in the subgroup aged ≤65 years (online supplemental additional file 1: fig.S2).
Joint effects of hsCRP and MetS on the risk of aICAS. The groups are defined as follows: 00—no metabolic syndrome, hsCRP in quartiles 1–3; 10—metabolic syndrome present, hsCRP in quartiles 1–3; 01—no metabolic syndrome, hsCRP in quartile 4; 11—metabolic syndrome present, hsCRP in quartile 4. Adjusted for age, sex, coronary heart disease, MHR and NLR. aICAS, asymptomatic intracranial arterial stenosis; hsCRP, high-sensitivity C reactive protein; MetS, metabolic syndrome; MHR, monocyte-to-high-density lipoprotein-cholesterol ratio; NLR, neutrophil-lymphocyte ratio.
Interaction between MetS and hsCRP with aICAS
We hypothesised that hsCRP might have interactive effects on the association between MetS and aICAS. However, the results were non-significant for both the additive and multiplicative scales (additive: RERI=−0.05, 95% CI −1.64 to 1.48; multiplicative, OR=0.82, 95% CI 0.39 to 1.75). We further conducted interaction analysis in the subgroup ≤65 years, but it also yielded negative results (additive: RERI=0.20, 95% CI −1.62 to 1.96; multiplicative, OR=0.90, 95% CI 0.39 to 2.07). Detailed results are shown in the online supplemental additional file 1: table.S4 and S5.
Mediation analysis
hsCRP did not mediate the relationship between MetS and aICAS in the overall sample (indirect effect: 0.002, p=0.126) or in the subgroup aged >65 years (indirect effect: −0.001, p=0.840). Notably, among individuals aged 65 years and younger, the mediation analysis demonstrated a significant partial mediation effect, where the inflammatory marker hsCRP mediated the association between MetS and aICAS (indirect effect: 0.004, 95% CI 0.00 to 0.01, p=0.044; adjusting for potential covariates). The average proportion mediated by hsCRP was 12.1% (table 4).
Discussion
In our study, we found that MetS is an independent predictor of aICAS risk. Additionally, both grouping MetS by the number of risk factors and categorising Metsss into quartiles yielded consistent results, demonstrating that a higher number of MetS components and a higher MetS severity score are associated with an increased risk of aICAS. Furthermore, metabolic disorder appears to be more closely associated with posterior aICAS. This finding is consistent with a previous multicentre prospective study on symptomatic ICAS, which reported a stronger association of posterior circulation stroke with MetS.25
In this neurologically healthy cohort, the prevalence of aICAS was 5.65%, which aligns with findings from a study in Japan, where 5.9% (166/2807) of neurologically healthy individuals were found to have aICAS via MRA.26 Compared with the Japanese study, our cohort had lower rates of hypertension, diabetes, hyperlipidaemia and smoking. The prevalence of aICAS varies across populations and diagnostic methods. Previous studies have compiled and compared the incidence of ICAS across Asia, Brazil, Europe and the USA. They reported that the incidence is highest in Asian countries, ranging from 9% to 65%, relatively lower in Europe and the USA at 10% to 16%, and around 39% in Brazil.3 A study using TCD in a rural area of Liangbei, China, among individuals aged 40 and above, reported an aICAS prevalence of 6.9% (41/590).27 Additionally, a study in urban Shandong, using TCD screening and MRA verification, reported a prevalence of 7.6%.28 Shuyuan Li et al.29 reported a 12.81% prevalence of aICAS via MRA among individuals aged 55 to 65 years in Taizhou. Our findings add to the epidemiological evidence of aICAS in the Chinese health check-up population.
In the diagnosis of aICAS, TCD has several limitations, including its dependence on the operator’s skill, difficulties in penetrating the bone window in some patients and challenges in assessing the posterior circulation.30 Studies have shown that approximately 8.2% of patients lack an adequate acoustic window, with failure rates increasing with age, sex and temporal bone thickness.31 32 MRA mitigates some of these limitations to a certain extent. The continuous severity score of MetS, although calculation methods vary, has been shown to identify individuals at higher risk for MetS-related diseases, such as cardiovascular disease and mortality,33 34 cerebral white matter hyperintensities and lacunes.35 Our findings are consistent with previous research. In 2021, Mei-Jun Shu et al demonstrated that the MetS severity Z- score is an independent risk factor for ICAS in a community-based cohort.35 Notably, their method did not account for diastolic blood pressure. Our study contributes new evidence on the role of Metsss in cerebrovascular research.
Evidence suggests that cerebral artery stenosis, even mild to moderate aICAS, is an independent predictor of future vascular events in stroke-free individuals,5 26 as well as recurrent stroke and patient prognosis.36 Our study indicates that MetS, an increasing number of MetS components, and a rising MetS severity score are associated with aICAS, suggesting that MetS control may be crucial for stroke primary prevention.
MetS patients are often obese and insulin resistant, experiencing systemic oxidative stress, which leads to elevated pro-inflammatory markers and chronic low-grade inflammation. Inflammation plays a critical role in the development of atherosclerotic cardiovascular disease.37 In this study, we observed a potential link between hsCRP and MetS, with the risk of aICAS being greatest when both MetS and elevated hsCRP levels are present.
On the basis of these findings, we hypothesised that the inflammatory marker hsCRP may have interactive effects on, or mediate, the association between MetS and aICAS. However, we did not find significant positive results. Our results align with another study, which reported that despite elevated hsCRP levels in MetS patients, hsCRP did not enhance the prediction of CVD risk in an older CVD-free population. The authors attributed this negative result to the older age of the participants (mean age: 72.00; SD: 6.00).24
As a result, we conducted a subgroup analysis based on age, focusing on participants aged 65 years and younger. Although the interaction remained non-significant, we found that hsCRP partially mediated the relationship between MetS and aICAS in this subgroup. This finding supports the notion that the additional impact of hsCRP on MetS is age-dependent, typically occurring in middle-aged populations. The underlying mechanisms warrant further investigation.
To the best of our knowledge, three studies have explored the association between MetS and aICAS: the Kongcun Town study,10 the Asymptomatic Polyvascular Abnormalities Community study9 and the Shunyi study.35 In comparison with these studies, our work provides novel insights. As shown in online supplemental Additional file 1: table.S6, unlike the first two studies, we employed MRA for screening and adopted the WASID criteria to define ICAS. Two imaging readers were uniformly trained, and Kappa consistency analysis ensured the reliability of the detection methods. Additionally, our study population differed from those in previous community-based studies. Our study primarily focused on the working population, who tend to be younger and exhibit lower MetS and aICAS detection rates. These demographic differences render our findings particularly relevant for guiding preventive strategies in these groups. Furthermore, our study distinguished between anterior and posterior circulation aICAS, revealing significant differences in metabolic burden between the two regions. These findings should inform the development of prevention and management strategies for aICAS. Notably, our study introduced mediation analyses of metabolic and inflammatory factors, thereby providing a scientific basis for formulating future preventive strategies.
Several limitations exist in our study. First, we excluded patients with a history of neurological diseases. However, theoretically, it might be more reasonable to include individuals with aICAS and a history of contralateral lacunar infarction. Second, the cross-sectional design of our study hinders us from inferring causation. Although our results demonstrated that hsCRP played a mediating role in the relationship between MetS and aICAS, the current evidence remains inadequate to establish causality. Concurrently, given that only a modest mediating effect was observed, further large-scale, multicentre and prospective cohort studies are necessary to validate our findings. Since we included a neurologically healthy population, the prevalence of aICAS was relatively low, which impeded our further exploration of associations between the number of arteries affected by aICAS, the severity of aICAS, and MetS. Finally, future research should incorporate high-resolution MRI to assess plaque stability, and longitudinal follow-up studies on individuals diagnosed with aICAS are essential for a better understanding of disease progression.
This study also has several strengths. First, the components of MetS can be easily obtained through routine questionnaires, physical examinations and laboratory tests, without additional cost, making them widely applicable in clinical practice. Second, we used MRA to asses intracranial atherosclerotic stenosis. This non-invasive imaging modality improves measurement accuracy and is suitable for general health check-ups. Third, our study excluded individuals with hsCRP levels ≥10 mg/L. This, to some extent, alleviates the impact of acute inflammatory responses. Theoretically, if the results for >10 mg/L could be remeasured within 2 weeks, the measurement would better reflect the person’s risk level. However, it is difficult and impractical to implement among health check-up populations. Finally, we identified the mediating role of hsCRP in the relationship between MetS and aICAS in individuals aged ≤65 years, which might potentially hold significance for aICAS risk management and stroke prevention across different age groups.
Mediation analysis of hsCRP in the association between MetS and aICAS in the total sample and the subgroup
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by IRB of Beijing Tiantan Hospital, Capital Medical University KY 2020-085-02. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors thank the study subjects for their participation and support of this study.
Footnotes
Contributors Jie L contributed to the study design, statistical analysis, preparation of tables/figures, MRA evaluation and manuscript writing. YL contributed to data acquisition, literature research and study design. LZ contributed to data acquisition and study design. WL contributed to MRA evaluation and study design. YZ, YH and Juan L contributed to data acquisition and organisation. YD reviewed the MRA evaluation. Jie L, HZ and YD reviewed and edited the intellectual content. HZ is the guarantor of the study’s integrity, contributing to the study design, literature research, statistical analysis, manuscript editing and final approval of the manuscript. All authors have given their final approval for this version to be published and have read and approved the final manuscript. The authors declare no potential competing interests.
Funding This study was supported by National Key Research and Development Program of China (2018YFC1311203).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.