Abstract
Background Malignant swelling is a fatal complication that can occur abruptly in space-occupying cerebellar infarction. We aimed to establish markers that predict malignant swelling in cerebellar infarction.
Methods We retrospectively analysed data of stroke patients who were treated in our hospital between 2014 and 2020. Malignant swelling was defined as a mass effect in the posterior cranial fossa, accompanied by a decrease in consciousness due to compression of the brainstem and/or the development of obstructive hydrocephalus. Statistical analyses were performed on multiple variables to identify predictors of malignant swelling.
Results Among 7284 stroke patients, we identified 487 patients with an infarct in the cerebellum. 93 patients were suitable for analysis having space-occupying cerebellar infarction. 33 of 93 (35.5%) patients developed malignant swelling. Multivariable analysis revealed infarct volume as the main predictor being independently associated with the development of malignant swelling with a cut-off infarct volume of 38 cm3 being associated with a swelling rate of >50% (OR 32.0, p<0.001). Higher NIHSS (National Institutes of Health Stroke Scale) score on admission (median NIHSS 12 vs 4, OR 1.078; p=0.008) and the presence of additional brainstem infarction (51.5% vs 16.7%, OR 5.312; p=0.013) were associated with the development of malignant swelling in univariate analyses. 13 of 33 (39.4%) cases of malignant swellings occurred after more than 3 days.
Conclusions Infarct volume was the key significant predictor of malignant swelling in space-occupying cerebellar infarction. With many cases of malignant swelling occurring after more than 72 hours, we advocate prolonged neurological monitoring.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patients with space-occupying cerebellar infarction are at increased risk of abruptly developing malignant swelling as a fatal complication.
However, data on incidence, risk factors and onset of malignant swelling are scarce.
WHAT THIS STUDY ADDS
In this study, we found that malignant swelling occurred in 35.5% of patients with space-occupying cerebellar infarction.
The key significant predictor of malignant swelling was infarct volume.
An infarct volume of 38 cm3 or more was associated with a swelling rate of over 50%.
Over a third of malignant swellings happened after more than 72 hours.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our study suggests prolonged neurological monitoring in patients with space-occupying cerebellar infarction, especially in cases with a large infarct volume.
Further research is needed to establish clear clinical practice guidelines.
Introduction
Cerebellar infarctions account for about 3% of strokes.1 While composing a small percentage of strokes, they constitute a disproportionately higher amount of mortality due to their sometimes subtle and non-specific initial presentation and rapid malignant swelling in the posterior fossa. Malignant swelling, resulting in compression of the brainstem and obstructive hydrocephalus, occurs in about 10–20% of patients with cerebellar infarction and represents a potentially fatal but treatable complication if recognised early.1 However, space-occupying cerebellar infarction has not received the same level of attention in medical publication as the well‐described malignant middle cerebral artery infarction. Therefore, the purpose of this study is to identify potential predictors for malignant swelling in cerebellar infarction.
Methods
This study was performed in line with the principles of the Declaration of Helsinki. The data that support the findings of this study are available from the corresponding author on reasonable request.
Data source, patient selection and cohort development
Patients who were admitted to the university hospital Klinikum rechts der Isar of the Technical University of Munich and diagnosed with acute cerebellar ischaemic stroke between 1 January 2014 and 31 December 2020 were eligible for inclusion in this retrospective study. We retrospectively identified patients with acute cerebellar ischaemic stroke from our stroke quality assurance registry (Bavarian Working Group for Quality Assessment, BAQ).
Study entry criteria was acute cerebellar infarct confirmed on either CT or MRI. From all patients with cerebellar infarctions, we selected those with a space-occupying effect on CT or MRI, defined as a mass effect in the posterior cranial fossa, for further detailed analysis. Patients with simultaneous large supratentorial stroke (infarction or haemorrhage) or extensive cerebellar haemorrhage were excluded from the study.
Patient and public involvement
Patients were not involved in determining study conduct, recruitment and design.
Baseline demographic, clinical and radiological characteristics
Patient demographic and clinical characteristics included age, sex, National Institutes of Health Stroke Scale (NIHSS) on admission, stroke subtypes according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria, intravenous thrombolysis with tissue plasminogen activator (tPA, alteplase) and endovascular treatment (EVT). Radiological parameters, such as infarct volume, brainstem compression, hydrocephalus, side of the infarction, vascular territory, basilar artery occlusion and the presence of additional brainstem infarction, were reviewed by experienced neuroradiologists. Infarct volume was measured using the well-known ‘ABC/2’ method,2 in which A is the largest diameter of the selected slice by eye with the largest infarct area, B is the largest diameter perpendicular to the line above and C is the total number of slices of infarct seen multiplied by the slice thickness. The infarct volume was assessed on the baseline CT or MRI, meaning cranial imaging conducted within the first 24 hours of symptom onset. In case there were multiple CT or MRI examinations available within the first 24 hours, the examination with the best quality was used, distinctly showing the demarcated infarction area. Brainstem compression was defined as CT or MRI evidence of distortion of the normal contour of the midbrain, pons or medulla oblongata by the cerebellar infarct.3
Endpoint
The primary endpoint was the development of malignant swelling defined as a mass effect in the posterior cranial fossa, accompanied by a decrease in consciousness due to compression of the brainstem and/or the development of obstructive hydrocephalus.4 Other potential underlying causes of decreased consciousness were ruled out.
In addition, we analysed the time span between symptom onset and development of malignant swelling.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows (V.28.0.1.1). The total cohort was divided into two groups: one with and the other without malignant swelling. Descriptive statistics were presented as frequencies with percentages for categorical variables, as means with SD for approximately normally distributed continuous variables and as median with range for ordinal or not normally distributed variables as appropriate. To identify potential predictors of malignant swelling, we used univariate and multivariable logistic models and presented the results using OR, its 95% CI and the p value of the Wald statistic. Time to swelling was analysed using the Kaplan-Meier method. All statistical tests were performed two-sided at the global 5% significance level. The Bonferroni-Holm correction was used to adjust the p values for multiple testing.
Results
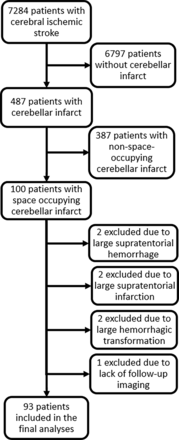
Our database encompassed 7284 patients with cerebral ischaemic stroke during the 7-year study period, of whom 487 had an infarct located in the cerebellum. The analysis of the cerebral imaging data identified 100 patients with space-occupying cerebellar infarction.
Seven patients were excluded due to simultaneous large supratentorial intracerebral haemorrhage (n=2), large supratentorial infarction (n=2), extensive haemorrhagic transformation of the cerebellar infarct (n=2) and lack of follow-up imaging despite clinical deterioration (n=1). Thus, 93 patients fulfilled all predefined criteria. Thirty-three of the 93 patients (35.5%) from the cohort developed malignant swelling according to imaging and clinical data retrieved from medical records. The patient selection flowchart is depicted in figure 1.
Patient selection flow chart.
Comparison of patients with and without malignant swelling
A comparison of baseline demographic, clinical and radiological characteristics is summarised in table 1. Among the clinical characteristics, higher NIHSS score on admission is associated with the development of malignant swelling. Comparing the median NIHSS score on admission demonstrated a higher risk for malignant swelling having a higher score (median (range) of NIHSS: 12 (1–38) vs 4 (0–36), OR 1.078 ((95% CI 1.034 to 1.124); adjusted p=0.008).
Comparison of demographic, clinical and radiological characteristics between patients with space-occupying cerebellar infarct with and without malignant swelling
Among imaging parameters, three constellations were identified as significantly associated with the development of malignant swelling: infarct volume presented with mean±SD: (61.8 cm3 (±17.1) vs 41.0 cm3 (±9.5), OR 1.136 (95% CI 1.078 to 1.196); p<0.001), infarctions in multiple different vascular territories (39.4% vs 15.0%, OR 3.683 (95% CI 1.362 to 9.961); p=0.010) and the presence of additional brainstem infarction (51.5% vs 16.7%, OR 5.312 (95% CI 2.028 to 13.914); p<0.001). Patients with a brainstem infarction (n=27) also had a significantly higher rate of basilar artery occlusion (66.7%) compared with those without infarct of the brainstem where only 8 out of 66 patients (12.1%) had a basilar artery occlusion (p<0.001).
On adjusting for multiple testing, brainstem infarction (p=0.013) and infarct volume (p<0.001) remained significant.
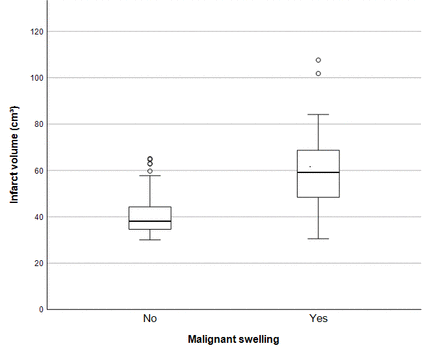
In our attempt to build a multivariable logistic regression model, only infarct volume showed a significant effect. A cut-off infarct volume of 38 cm3 or more was associated with a malignant swelling rate of >50% (97.0% vs 50.0%, OR 32.0 (95% CI 4.1 to 249.5); p<0.001). All patients are depicted according to their infarct size in figure 2.
Box plot showing the distribution of infarct volume (cm3) in both groups.
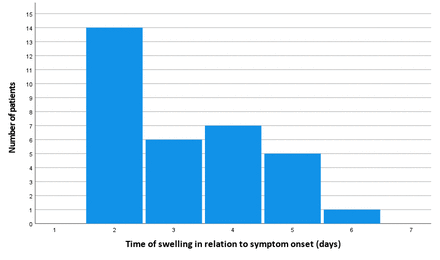
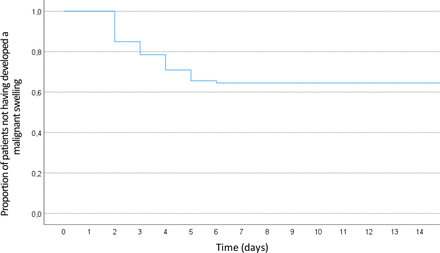
In addition, we analysed the time between symptom onset and the occurrence of malignant swelling, as illustrated in figure 3. All malignant swellings of the cerebellum happened within the first week after stroke occurrence, median on day 3, meaning 48–72 hours after symptom onset. Kaplan-Meier analysis, as depicted in figure 4, was used to model cumulative probability for malignant swelling.
Day of occurrence of malignant swelling in relation to symptom onset.
Kaplan-Meier curve showing the cumulative probability of not developing malignant swelling in all patients.
Discussion
In this retrospective single-centre study, we found that a larger infarct volume, higher admission NIHSS and the presence of additional brainstem infarction are associated with the development of malignant swelling in patients with space-occupying cerebellar infarction.
While several studies examined the clinical outcome of space-occupying cerebellar infarcts5 or the timing and effect of a decompressive therapy,6–9 there have been no studies analysing the potential predictors of malignant swelling. With the devastating course of malignant swelling in space-occupying cerebellar infarcts being well-known,1 we focused on the occurrence of malignant swelling as the primary endpoint. A large infarct volume being the main significant predictor for malignant swelling is in accord with the results from studies about malignant middle cerebral artery infarction like the DIRECT-MT trial10 and another prospective multicentre study about predicting the emergence of malignant brain oedema in middle cerebral artery infarction.11
However, infarct volume plays an even more important role in cerebellar infarction than in middle cerebral artery infarction considering the tightly constrained posterior fossa and the close proximity of the cerebellum to the brainstem. It is well known that cerebellum and brainstem are bounded above by a rigid dural reflection (tentorium cerebelli) and below by the base of the skull,1 leaving only little space for an oedema to expand before it causes a compressing effect on the brainstem and obstructs the flow of cerebrospinal fluid resulting in hydrocephalus.
Nevertheless, as not all patients with large infarction have a malignant course, other clinical and radiological features are needed to be examined.
With regard to acute stroke severity indices, we chose the NIHSS score. While it is known for rather underestimating the severity of ischaemic stroke in the cerebellum with symptoms of the posterior circulation like vertigo, nystagmus and imbalance of gait not being part of the score,12 13 it is still one of the most reliable and valid tools to evaluate neurological impairment in patients with stroke.14
The association between the presence of an additional brainstem infarction and malignant swelling can be attributed to the fact that the basilar artery supplies both the upper brainstem and a large part of the cerebellum.15 The fact that an occlusion of the basilar artery was present in two-thirds of patients with a brainstem infarction but only in 12% of patients without supports this assumption.
The fact that all five cases of failed revascularisation of basilar artery occlusion resulted in malignant swelling is plausible considering that any acute obstruction of the basilar artery will ultimately lead to cutting off vital vascular supply to large parts of the infratentorial region, although the statistical power is overall low due to a small sample size with only 26 basilar artery occlusions, 24 endovascular treatments and 5 failed revascularisations.
In addition, the analysis of the time span between symptom onset and space-occupying effect of the infarction showed all malignant swellings happening between day 2 and day 6. This coincides with findings from studies on malignant middle cerebral artery infarction describing a progression of cerebral oedema after acute infarction between 2 and 5 days.16 17 With over a third of malignant swelling occurred more than 3 days after symptom onset, our data support that patients with space-occupying cerebellar infarction should receive longer, more continuous and closer neurological monitoring than the average stroke patients with usually monitoring of 72 hours according to the European Stroke Organisation Recommendations.18
The main limitations of our study are owed to the retrospective and single-centre design.
Our analyses depend on data from medical records that were not primarily collected for research purposes. Despite a large stroke database with over 7000 patients who had ischaemic stroke in a 7-year period, only 93 patients could be included in the final analyses according to predefined inclusion and exclusion criteria. This rather small cohort size therefore has a limited statistical power. However, the main strength of our study is that we provide a homogeneous dataset from a real-world clinical practice setting of patients who may not have been included or may have been under-represented in prospective trials. Our findings provide valuable clinical insights into a topic that has not yet been sufficiently explored.
In conclusion, our study demonstrates that infarct volume plays a key role in predicting malignant swelling in space-occupying infarctions. Higher baseline NIHSS score and additional brainstem infarction were also associated with malignant swelling. While the median day of swelling was day 3, 39.4% of malignant swelling occurred more than 3 days after symptom onset. Therefore, longer neurological monitoring as usual should be suggested in this critically ill patient population. Prospective, multicentre studies with larger patient cohorts are required to reproduce and validate our findings prior to clinical implementation of the ideal timeframe of neurological monitoring and also possible treatment or preventive strategies.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Ethical approval was granted by the local ethics committee (2022-96_1-S-SB).
Footnotes
Contributors EB is responsible for the overall content as guarantor. EB, AWW, BM, JG and SW developed the theory and designed the study. EB, LB and AW collected and processed the clinical patient data. MRHP and TB-B. analyzed imaging data. VK performed the statistical analyses and created the figures. EB wrote the manuscript with support from VK, FH, BH and SW. All authors discussed the results and contributed to the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.