Abstract
Objectives Despite the potential spillover effect, the optimal duration of dual antiplatelet therapy for minor stroke within 72 hours of symptom onset is still uncertain.
Methods Safety and Efficacy of Aspirin-Clopidogrel in Acute Noncardiogenic Minor Ischemic Stroke (National Institutes of Health Stroke Scale (NIHSS) score≤5) is a prospective cohort study involving patients with minor ischaemic stroke within 72 hours of symptom onset. The DAPT group was further categorised into three subgroups: shorter duration (<10 days), short duration (10–21 days) and long duration (>21 days). The primary efficacy and safety outcomes were composite vascular event and severe bleeding during 90 days.
Results Among 3061 eligible patients (age was 61.7±12.0 years, 73.3% were men, median (IQR) NIHSS score, 2 (1–3)), 2977 (97.4%) completed the follow-up. Dual antiplatelet therapy (DAPT) and single antiplatelet therapy (SAPT) were administered in 61.0% and 39.0% of patients. Among them, 305 patients (16.8%) received a shorter duration of DAPT, 937 patients (51.7%) received a short duration and 572 patients (31.5%) received a long duration. In the propensity-weighted Cox proportional hazards regression analysis, the use of DAPT in the short-duration group was associated with a lower risk of the primary vascular event outcome (HR (HR)=0.66, 95% CI 0.46 to 0.94, p=0.02) compared with SAPT group. The incidence of severe bleeding events at 90 days was similar. Similar findings were obtained from the propensity score-matching analysis.
Conclusion Short duration of DAPT (10–21 days) is superior to SAPT in minor stroke within 72 hours, reducing 90-day composite vascular events without increasing bleeding risk.
WHAT IS ALREADY KNOWN ON THIS TOPIC?
Dual antiplatelet therapy (DAPT) within three days of stroke symptom onset may still be beneficial.
The effectiveness of DAPT in terms of spillover effects within 72 hours of stroke symptom onset remains unclear, and the optimal duration remains uncertain.
WHAT THIS STUDY ADDS?
This prospective cohort study showed that a short duration of DAPT could reduce the risk of composite vascular events by 34% at 90 days.
No significant difference was observed in the incidence of severe bleeding events between the two treatment groups.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY?
This study provides evidence that minor stroke patients may derive significant benefit from a short duration of DAPT within 72 hours of symptom onset without an associated increase in the risk of bleeding.
Introduction
Transient ischaemic attack (TIA) and mild stroke account for 65% of ischaemic strokes and they are both prone to early recurrence.1 The American Heart Association/American Stroke Association (AHA/ASA) recommends dual antiplatelet therapy (DAPT) for patients with acute minor stroke (National Institutes of Health Stroke Scale (NIHSS) score ≤3) or high-risk TIA started within 12 or 24 hours of symptom onset.2–4 A time-course analysis combining the Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (PIONT) and Clopidogrel in High-risk Patients with Acute Non-disabling Cerebrovascular Events (CHANCE) studies suggested an optimal 21-day DAPT duration.5 6 The benefits mainly manifest within 10 days, with limited or no reduction in stroke risk thereafter.7 Prolonging DAPT beyond this acute phase may increase bleeding risk without added benefits.6
Previous studies have indicated that starting DAPT within 72 hours of stroke symptom onset might still be beneficial,8 9 with real-world studies showing a substantial spillover effect.10 In Korea, approximately one-third of mild stroke and patients with TIA receive DAPT within 48 hours.11 The risks and benefits of the ‘indication creep’ phenomenon, whereby the benefits of DAPT are uncertain in non-minor stroke patients, has not been resolved. The definition of mild stroke varies, with CHANCE and POINT using NIHSS ≤3, while thrombolysis and endovascular studies often consider it to mean NIHSS ≤5.3 4 12 13 The China Stroke Centre Alliance (CSCA) reported comparability of in-hospital outcomes between patients with NIHSS scores ≤5 and ≤3.14 Despite limited evidence, more than 40% of non-minor stroke patients receive DAPT according to the AHA/ASA study.15 Additionally, the Acute Stroke or TIA Treated With Ticagrelor and Acetylsalicylic Acid for Prevention of Stroke and Death trial indicated potential benefits of DAPT in patients with ischaemic stroke (NIHSS 4–5) started within 24 hours.16 17
Therefore, we hypothesise that the spillover effect of DAPT with aspirin and clopidogrel can extend to patients with NIHSS scores between 0 and 5 and within 72 hours and that a short duration of DAPT may be the most effective and safe treatment. We attempted to validate this hypothesis through a real-world cohort study.
Methods
Study design, participants and setting
Safety and Efficacy of Aspirin-Clopidogrel in Acute Noncardiogenic Minor Ischemic Stroke (SEACOAST) (ChiCTR1900025214) is a prospective, real-world multicentre cohort study involving patients admitted to eight stroke centres in Shanxi Province, China, from 1 September 2019 to 31 November 2021. The protocol is found in online supplemental materials. Informed consent was obtained from the patients or their guardians.
Supplementary data
(The content marked red in the article is the revision comments for review, it should be change block color. Thank you very much!)The inclusion criteria were as follows: (1) onset of stroke symptoms to hospital admission time ≤72 hours (the time of last known normal was defined as the start of symptoms); (2) baseline NIHSS score ≤5 points; (3) treatment with oral aspirin, clopidogrel or a combination of aspirin and clopidogrel and (4) signed informed consent. The detailed inclusion and exclusion criteria are provided in online supplemental materials. This study followed the guidelines outlined in the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.18
Sample size calculation
We identified significant gaps in specific data regarding stroke recurrence rates within the target population before commencing this prospective study. Previous studies have shown higher primary outcome event rates and a corresponding need for smaller sample sizes among Asian populations. Therefore, we calculated the sample size based on single-centre retrospective data.
Our single-centre study results predicted that the probability of stroke recurrence was approximately 10.8% in the aspirin group and 6.1% in the DAPT group. Considering the 10.8% recurrent stroke rate in the aspirin group and the 6.1% recurrent stroke rate in the DAPT group, a p value <0.05, indicating statistical significance at the 95% CI, and a 10% dropout rate, the target number of participants is at least 2500; PASS V.22.0 was used to perform the study.
Data collection
The data were captured and handled utilising the Research Electronic Data Capture (REDCap) system.19 All researchers underwent training before data collection. We collected comprehensive demographic data, clinical characteristics, imaging results and laboratory findings. The detailed data collection process is described in the protocol.
Patients were classified into two groups based on their initial antiplatelet treatment on hospital arrival: single antiplatelet therapy (SAPT), which involved either aspirin or clopidogrel, and DAPT, which consisted of aspirin combined with clopidogrel. Antiplatelet treatment commenced within a few hours of admission and continued for 90 days or less. In the DAPT group, patients received a combination of 75–300 mg of clopidogrel and 100–300 mg of aspirin on the first day, followed by guideline-based antiplatelet treatment for 90 days, and in the SAPT group, patients received a daily dose of 75 mg of clopidogrel or 100 mg of aspirin for 90 days. The treating physicians determined the administration schedule and dose adjustments based on careful consideration and patient preferences. The types and doses of the different therapies are provided in online supplemental table 1. Three trained investigators, blinded to the assigned treatments, independently assessed the occurrence of efficacy outcome events and bleeding events, whichever occurred first.
Supplementary data
(The content marked red of sTable 19 and sTable 20 in the article is the revision comments for review, it should be change block color. Thank you very much!)To determine the duration of medication use, we calculated the difference between the time of first DAPT administration and the time of confirmed DAPT cessation during the follow-up period. Based on predefined follow-up time points, the DAPT group was further divided into three groups: DAPT duration less than 10 days, DAPT duration between 10 and 21 days and DAPT duration greater than 21 days. Each subset was compared with the SAPT group.
Clinical outcomes
The primary outcome was a composite vascular event (including ischaemic stroke recurrence,20 TIA,21 symptomatic intracerebral haemorrhage,22 myocardial infarction or angina attacks23 and vascular death24) occurring during the 90 days of follow-up. Secondary outcomes included any vascular event during the 90 days of follow-up, with the definition provided in the Methods section in online supplemental materials. Safety endpoints involved severe bleeding (defined by the Global Use of Strategies to Open Occluded Coronary Arteries)25 during the follow-up period.
All patients were followed up on days 1, 10, 21 and 90±7 postenrolment. The same neurologist assessed both the baseline and follow-up NIHSS scores. Trained personnel used uniformly structured questionnaires via telephone to complete the follow-up.
Patient and public involvement
All participants were provided with an overview of the trial’s objectives and specifics during recruitment, although they were not involved in the development of the research question, the selection of the outcome measures or the planning and execution of the study design. The research findings will be disclosed to the patients and the public through press releases and subsequent communications following the publication of the study report.
Statistical analysis
Statistical analyses were conducted on a full analysis set comprising all participants who completed the 90-day follow-up. The specifics of the statistical analysis are outlined in online supplemental materials.
To mitigate treatment selection bias and account for potential confounders in our observational study, we used rigorous adjustments for patient characteristics through weighted Cox proportional hazards regression models employing inverse probability of treatment weighting (IPTW). Propensity score matching was also conducted to validate the analysis results obtained through IPTW. Nearest neighbour matching with a calliper width of 0.2 of the propensity score was employed for a 1:1 matching ratio. Kaplan-Meier failure curves illustrating event distributions in stratified groups, with the log-rank test generating corresponding p values, were used to analyse the primary outcome. For interaction testing, evidence of heterogeneity was considered present with p values≤0.1.
A two-sided p value of less than 0.05 was considered to indicate statistical significance for the secondary outcomes. Statistical significance was generally determined using 95% CIs and two tailed p values (p≤0.05). All analyses were performed in SPSS V.26.0 and the statistical software R V.4.1.2 (http://www.R-project.org, The R Foundation).
Results
General characteristics
Between 1 September 2019 and 31 November 2021, an initial screening identified 3723 patients, 3061 of whom met the eligibility criteria. After excluding 84 patients lost to follow-up, the full analysis population consisted of 2977 patients (1163 in the SAPT group and 1814 in the DAPT group; figure 1).
Flowchart of the study. NIHSS, National Institutes of Health Stroke Scale; rt-PA, Recombinant human tissue type plasminogen activator.
Among the 2977 patients with acute, minor or non-cardiogenic ischaemic stroke, the mean age was 61.7±11.9 years, 73.3% were men and the median National Institutes of Health Stroke Scale score was 2 (IQR, 1–3). The characteristics of the patients who received SAPT (n=1163, 39.0%) and those who received DAPT (n=1814, 61.0%) are detailed in online supplemental table 2. DAPT durations were distributed as follows: shorter duration (<10 days), 16.8%; short duration (10–21 days), 51.7%; and long duration (>21 days), 31.5%) (online supplemental table 3). Additional details are found in online supplemental tables 2–6. Following IPTW and propensity score matching, the baseline characteristics were well balanced, with absolute standardised differences within a margin of 0.1 for most covariates (online supplemental tables 2–6). Patients lost to follow-up were assumed to be event free for the primary outcome analysis.
Efficacy and safety outcomes
The mean follow-up duration was 83.4±22.3 days, and 97.4% of patients completed 90 days of follow-up. Composite vascular events occurred in 257 patients (8.0%), with individual component rates within 90 days as follows: 6.7% for ischaemic stroke, 0.7% for TIA, 0.2% for symptomatic intracranial haemorrhage, 0.2% for acute myocardial infarction and 0.1% for vascular death. The overall bleeding event rate was 6.5%, with 0.2% classified as moderate to severe bleeding and 2.0% as intracranial haemorrhage. Unadjusted analysis did not reveal statistically significant differences between the two groups. However, in the propensity analysis using IPTW, the DAPT group had lower rates of primary outcome events, ischaemic stroke, TIA and all ICH compared with the SAPT group (online supplemental tables 7,8). The weighted absolute risk differences for these outcomes were 1.7%, 1.4% and 0.3%, respectively. Similar results were observed in the sensitivity analysis using standardised mortality ratio weighting . Detailed information is available in online supplemental tables 9,10.
Different durations of DAPT
Restricted cubic spline analysis suggested that there might be a ‘U-shaped’ association between DAPT duration and the primary outcomes (p for non-linearity=0.004) (online supplemental figure 1). According to the unadjusted analysis, patients who received DAPT for 10–21 days exhibited a decrease in the 90-day risk of composite vascular events (6.8% for DAPT vs 8.9% for SAPT, p=0.09) and ischaemic stroke (5.7% for DAPT vs 7.6% for SAPT, p=0.08). Propensity analysis using IPTW consistently demonstrated a lower incidence of major vascular events and ischaemic stroke in the DAPT group, with adjusted absolute risk differences of 3.0% and 2.9% and relative risks of 0.66 (95% CI 0.46 to 0.94, p=0.02) and 0.64 (95% CI 0.43 to 0.94, p=0.02), respectively (tables 1 and 2). Propensity score matching yielded consistent results for the primary outcome at 90 days (absolute risk difference of 6.1%, HR of 0.47, 95% CI, 0.27 to 0.82) and for ischaemic stroke (absolute risk difference of 4.7%, HR of 0.39, 95% CI 0.21 to 0.72).
Supplementary data
(The content marked red of sFigure5 in the article is the revision comments for review, it should be change block color. Thank you very much!)Efficacy outcome event rates* of single antiplatelet therapy and dual antiplatelet therapy with aspirin-clopidogrel in patients with short duration
HRs of dual antiplatelet therapy compared single antiplatelet therapy according to the analytical methods used in patients with standard duration
For patients receiving shorter or long durations of DAPT, the 90-day risk of composite vascular events was numerically higher in the DAPT group than in the SAPT group (shorter duration: 10.2% vs 8.9%, p=0.480; long duration: 10.4% vs 8.9%, p=0.326). However, no significant differences were observed in the efficacy analysis (online supplemental tables 11–14). Severe haemorrhage occurred in three patients (0.3%) in the SAPT group and one patient (0.1%) in the DAPT group (p=0.43) (table 1). The overall rate of any bleeding event was 6.2% in the short-duration DAPT group compared with 6.7% in the SAPT group (HR of 0.92, 95% CI 0.66 to 1.3; p=0.65) (online supplemental tables 7,8).
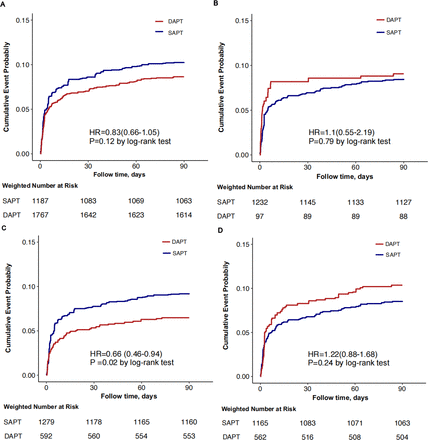
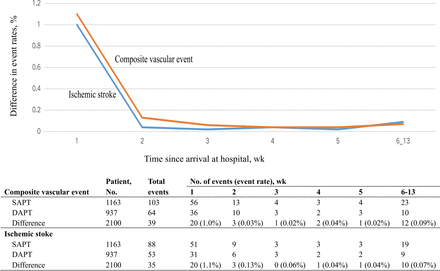
The weighted Kaplan-Meier cumulative incidence plots for the primary outcome and ischaemic stroke (figure 2A‒D) indicated that differences in outcomes emerged within the first 7 days of initiating therapy and remained consistent thereafter (figure 3). These differences in outcomes were consistent between the DAPT and SAPT groups at 90 days.
Cumulative probability of primary events. (A) The entire population; (B) population with brief duration (<10 days) of DAPT; (C) population with short duration (10–21 days) of DAPT; (D) population with long duration (>21 days) of DAPT. DAPT, dual antiplatelet therapy; SAPT, single antiplatelet therapy.
Time course of the absolute treatment difference. DAPT, dual antiplatelet therapy; SAPT, single antiplatelet therapy.
Subgroups and sensitivity analysis
Subgroup analysis revealed a treatment interaction with primary vascular events in patients with a history of ischaemic stroke, suggesting a greater benefit in those without such a history (hazard ratios: 0.65 and 1.18; p interaction=0.08; figure 4) within the 10–21 days DAPT group. The reduction in combined vascular events with DAPT remained consistent across the other major subgroups. Detailed subgroup analyses for the total population and different DAPT durations are found in online supplemental figures 2–4.
HR for the primary outcome in subgroups for population with short duration. DAPT, dual antiplatelet therapy; IPTW, inverse probability of treatment weighting; SAPT, single antiplatelet therapy; LAA, large-artery atherosclerosis; TOAST, Trial of Org 10172 in Acute Stroke Treatment; SVO, small-vessel occlusion; OE, stroke of other determined etiology; UD, stroke of undetermined etiology.
To further validate our findings, we conducted the same analysis on patients who did not qualify for randomised controlled trials (RCT) enrolment (online supplemental table 15). According to the propensity analysis using IPTW, a lower incidence of major vascular events persisted with DAPT than with SAPT, with an adjusted absolute risk difference of 4.2% and a relative risk of 0.54 (95% CI 0.31 to 0.93; p=0.03). Consistent results were observed in the propensity score-matching model (online supplemental tables 16–18).
Furthermore, a long-term secondary analysis of antiplatelet prevention revealed that 470 patients (16.2%) used DAPT as maintenance therapy (online supplemental table 19). According to the Cox model, compared with patients who discontinued any antiplatelet therapy, DAPT did not demonstrate superior efficacy compared with aspirin or clopidogrel monotherapy (HRs for DAPT and Clopidogrel Monotherapy (CM) were 1.95 and 1.86, respectively, p<0.0001) (online supplemental figure 5).
Discussion
This real-world study explored the potential spillover effect of DAPT in patients with mild non-cardiogenic ischaemic stroke (NIHSS score ≤5) within 72 hours of symptom onset. A short duration (10–21 days) of DAPT could reduce the risk of 90-day composite vascular events. These results remained robust even after adjusting for confounding factors in matched cohorts.
Our study defined minor stroke as an NIHSS score ≤5, which differs from the CHANCE and POINT studies but aligns with the Acute Stroke or Transient Ischaemic Attack Treated With Ticagrelor and ASA for Prevention of Stroke and Death (TALASE) study.3 4 16 The lack of a unified standard for defining minor stroke contributes to this discrepancy. An analysis conducted by the CSCA revealed that the use of NIHSS scores ≤5 and ≤3 to define minor stroke showed comparability.14 Our study included patients with minor stroke treated within 72 hours of onset, while the guidelines recommend initiating DAPT within 24 hours. A study from Korea showed that many patients who presented beyond 24 hours still received DAPT, although their study did not find any benefit in this subgroup.26 A meta-analysis showed that DAPT within 7 days can still benefit patients with ischaemic stroke.27 Modelling analysis from a cohort study of 15 000 individuals indicated that the optimal timing for starting DAPT might be within 72 hours after onset,28 which validates the rationale of our study design and the importance of verifying the spillover effect of DAPT in this population. The Intensive Statin and Antiplatelet Therapy for High-risk Intracranial or Extracranial Atherosclerosis (INSPIRES) trial also verified the spillover effect in the above population.29
Our study found that 257 patients (8.6%) with minor stroke experienced composite vascular events within 90 days, with 225 patients (7.6%) experiencing ischaemic stroke events within 90 days. A Korean cohort study, similar to the CHANCE trial, showed that the 90-day stroke recurrence rates for patients with NIHSS scores ≤3 treated within 24 hours of onset were 9.65%3 and 11.1%,30 respectively. These rates were higher than our study results, which could be attributed to the difference in patient recruitment periods. The CHANCE trial recruited patients between 2009 and 2012, while the Korean cohort study analysed data from 2008 to 2016, and our study analysed data from 2019 to 2021. With advancements in medical care and continuous updates to guidelines, the recurrence rate of stroke after standard treatment has gradually decreased.31 This could be a significant reason for the lower recurrence rate observed in our study. Our results are similar to those of the INSPIRES trial in China, which indicated that a new stroke occurred within 90 days in 222 patients (7.3%) in the clopidogrel–aspirin group and 279 patients (9.2%) in the aspirin group.32
Our results show that DAPT treatment may reduce the risk of major vascular events without increasing the risk of bleeding, which is consistent with the findings of the CHANCE trial, especially in patients who received DAPT for between 10 and 21 days. The incidence rates of major safety events were comparable to those observed in the CHANCE and POINT trials.
The INSPIRES study revealed that patients treated with DAPT for no more than 21 days had a lower risk of new stroke at 90 days than patients treated with aspirin therapy alone but had a low but greater risk of moderate-to-severe bleeding.32 In our study, the rate of severe bleeding appeared to be greater in the SAPT group than in the DAPT group, which may be related to real-world clinical decision-making: patients in the SAPT group may have inherently greater bleeding risks (online supplemental table 2) and thus doctors avoid using DAPT. In addition, our study revealed significant variation in the use and dosing of DAPT in a real-world setting (online supplemental table 20), which differs from the findings of RCTs,3 4 17 especially for the initial doses of 75 mg and 300 mg clopidogrel. Low-load doses may be associated with a lower risk of bleeding, similar to what was found in the CHANCE, POINT and INSPIRES studies.3 4 29
Although the POINT and CHANCE studies revealed that the earlier DAPT was initiated, the greater the benefit, mild strokes have the highest risk of recurrence within the first 3 weeks of onset.5 The time-dependent analysis conducted in the CHANCE and POINT studies revealed that the high risk of recurrent stroke in mild stroke patients is most pronounced within the first 3 weeks after stroke onset and then decreases significantly.9 Furthermore, a duration–benefit analysis revealed that the optimal treatment time for DAPT was 21 days, with a net benefit exceeding the effect of DAPT continued for more than 90 days.9 This means that a 21-day course of DAPT can reduce the risk of recurrent stroke without increasing the risk of bleeding.33 This finding is consistent with our findings (online supplemental figure 1). This implies that the spillover effect of DAPT depends on its duration, and short-term DAPT is the optimal approach for benefiting this population.
Further analysis of the effectiveness of secondary preventive treatment further confirmed this result: long-term DAPT did not have a superior effect compared with switching to SAPT after a short course of DAPT. This finding aligns with findings from studies such as the CHANCE and POINT trials, where visual inspection indicated that the benefits of DAPT are limited to the initial 21 days, and potential harm accumulates with ongoing clopidogrel use.7
The subgroup analysis showed that patients in the no-RCT population seemed to benefit more from DAPT, which might be because more patients were treated within 24–72 hours. It is possible that our study had an insufficient sample size to clearly distinguish these periods. To find the best time for treatment, in-depth research is essential; at the same time, we also need to explore the optimal duration of DAPT to maximise the balance of risks and benefits.
The Trial of Org 10172 in Acute Stroke Treatment TOAST) subgroup analysis revealed that DAPT may benefit patients with large artery atherosclerosis and other aetiologies, which is consistent with the findings of the CHANCE, POINT and INSPIRES studies.4 32 34 Clinical adjustments may be considered based on the results of cerebral vascular examination.35 The possibility of refining the duration of acute DAPT post hoc is intriguing. The INSPIRES study included patients who were treated within 72 hours of symptom onset and was designed with the idea that the duration of treatment with DAPT should not exceed 21 days.32 The results of the time effect analysis of this trial are expected in future.
Limitations
Our study had several limitations. First, our study did not observe significant differences in the entire population, which is related to our inadequate sample size. Compared with the total population of similar studies such as INSPIRES, our sample size is notably insufficient, limiting the conclusions we can draw. Therefore, in our interim analysis, we attempted to increase the sample size by extending the study period. However, during the time-effect analysis, we found significant differences in the short duration of DAPT, similar to the CHANCE trial, within our population. Encouragingly, our findings yielded meaningful conclusions in preadjustment and postadjustment and matching models, complementing RCTs. Second, our efficacy analysis assumed that patients lost to follow-up did not experience endpoint events, which may introduce a potential risk of inflated effect estimates. Third, our study population consisted solely of Chinese patients who had a mild stroke in Asia, limiting the representativeness and warranting cautious interpretation of the results, and it remains to be investigated whether the results are reproducible in other racial populations or countries. Nevertheless, our research opens new horizons for the secondary prevention of mild stroke within 72 hours of symptom onset. The findings also call for randomised trials to determine the optimal DAPT dose and duration to prevent the recurrence of stroke, which may help physicians choose the most effective antiplatelet therapy strategies to alter clinical practice in the future.
Conclusions
In conclusion, the SEACOAST study revealed that among patients with minor ischaemic stroke treated within 72 hours of symptom onset, a short duration (10–21 days) of DAPT is superior to SAPT for decreasing the risk of composite vascular events and ischaemic stroke at 90 days without increasing the risk of bleeding.
Supplementary data
Supplementary data
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The corresponding author is responsible for the data in this study.
Ethics statements
Patient consent for publication
Ethics approval
Informed consent forms were signed by the patients or their guardians. The procedure was approved by the Ethics Committee of the First Hospital of Shanxi Medical University, and approval was obtained from all other participating centres. The ethics approval number is 2019-SK004. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors would like to express our gratitude to all participating clinicians, all study participants, and their relatives at the eight centres of the SEACOAST study.
Footnotes
Contributors Conceptualisation: TL and Xiaoyuan Niu. Methodology: TL and YW. Investigation: TL, KZ and HF. Writing—original draft: LT. Writing—reviewing & editing: TL and Xiaoyuan Niu. Supervision: Xiaoyuan Niu. Guarantor: Xiaoyuan Niu.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer-reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.