Abstract
Vascular dementia (VaD) is the second leading cause of dementia after Alzheimer’s disease (AD). In comparison to AD, there is a decline in the incidence of VaD due to recent improvements in cardiovascular risk factors. Brain hypoperfusion and hypoxia due to vascular pathologies have been postulated as the primary disease mechanism of VaD. However, other factors such as neuroinflammation may also contribute to the development of VaD. Non-modifiable and modifiable risk factors have been attributed to VaD. The clinical features overlapping between AD and VaD create significant challenges for physicians. Newly developed biomarkers may potentially help differentiate VaD from other forms of dementia. Unlike AD, there is no Food and Drug Administration-approved drug or device for treating VaD. Current treatment options mainly target symptoms rather than slowing the development or progression of VaD. There are ongoing research studies testing the efficacy of various therapeutic strategies for VaD. In this narrative review, we will summarise current findings on epidemiology, attributed risk factors and disease mechanisms, as well as emphasise the importance of optimising lifestyle modifications and comorbid condition management in preventing or slowing down the development of VaD. Finally, current therapies and ongoing research studies of novel therapeutic interventions such as stem-cell therapy and neuromodulation are highlighted.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Vascular dementia (VaD) is the second most common cause of dementia with cognitive deficits followed by vascular insults. The heterogeneity of pathological aetiologies and clinical manifestations of vascular cognitive impairment (VCI) spectrum poses significant challenges in diagnosis and treatment development.
WHAT THIS STUDY ADDS
This review provides an updated summary of VCI and VaD incidence and prevalence, risk factors particularly recent advancements in genetic risk factors of VaD, disease mechanisms including a proposed cascade of molecular and cellular changes induced by vascular insults, potential biomarkers and diagnostic criteria, as well as treatment strategies.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
In this study, we provide discussions on work needed in each section with the hope to guide future research to better understand VaD pathogenesis and develop novel diagnostic biomarkers and therapeutic interventions targeted at VCI and/or VaD.
Introduction
As the second most common cause of dementia, vascular dementia (VaD) has consistently remained a worldwide health concern. VaD is classified as the emergence of cognitive deficits as a consequence of vascular events, such as stroke, atherosclerosis and heart disease.1 Vascular cognitive impairment (VCI) was introduced to include a broader spectrum of vascular insult-induced cognitive impairment conditions including mild, major and mixed forms as well as at-risk stage, with VaD classified as the most severe form of VCI.1 The heterogeneity of pathological aetiologies and clinical manifestations of the VCI spectrum poses significant challenges in diagnosis and treatment development. For example, mixed neurodegenerative and cerebrovascular pathologies, present in the majority of dementia patients, implicate an overlapping concept between VaD and Alzheimer’s disease (AD), as well as potential difficulties to categorise these two diseases separately. Frequent reclassifications of VCI and VaD further introduce inconsistency in diagnoses.1 While more research into treatment strategies targeted at VCI and VaD is imperative, this has often been overlooked considering the field’s prioritisation in risk modification and stroke prevention. The narrative review will provide an updated summary of VCI and VaD incidence and prevalence, risk factors, disease mechanisms, potential biomarkers, diagnostic criteria and treatment strategies with discussions on future work needed in the VaD research field.
Epidemiology
In the Framingham Heart Study, the decline in the incidence of VaD (p=0.004 for trend) was more rapid than that of AD (p=0.052).2 Similarly, a recent systematic review of population-based cohort studies analysed data from 27 studies and reported a consistent decrease in the dementia incidence over time in the USA and Europe.3 Coinciding with the declining trends, participants had improvements in most cardiovascular health factors including reductions in stroke, atrial fibrillation (AF), congestive heart failure and cerebral small vessel diseases (SVD).2 4
While the majority of the participants in the Framingham Heart Study are from European ancestry, different trends have been reported for other ethnicities. A cross-sectional study in Japan conducted in 1997–2016 reported a significant increase in AD-related dementia prevalence (p<0.05), but not in VaD (p=0.77).5 In a 2021 study in northern rural China, the incidence of overall dementia was 22.48 per 1000 person-years for dementia, 13.28 for AD, and 5.67 for VaD, which was higher than in other regions.6 In addition, increases in incidence and prevalence of dementia in Japan and incidence in Nigeria were reported.3 The implementation of public health policies addressing attributable lifestyle and comorbid risk factors of VaD and VCI may contribute to differences in incidence and trends over years in different ethnic and socioeconomic backgrounds.
Risk factors
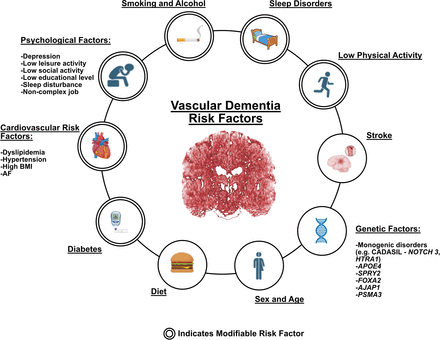
Multiple non-modifiable and modifiable risk factors have been attributed to the development of VCI and VaD (figure 1). Non-modifiable risk factors include age, sex and genetic risk factors. The incidence of dementia and vascular impairments is strongly correlated with age. The risk of vascular disorders for incident dementia decreases with age in late-life dementia patients. A recent study found that although most vascular disorders were risk factors for dementia at age 65–70 years, they were not significantly associated in individuals over 90 years.7 Sex differences in VaD remained less clear in recent studies.1 Men are thought to be more likely affected by VaD than women, presumably correlated to their higher risks for stroke.8 However, a 2016 meta-analysis on 2.3 million participants and over 100 000 dementia cases reported that women with diabetes had a 19% greater risk for VaD than diabetic men.9
Risk factors attributable to the development of VCI and VaD. Non-modifiable risk factors include age, sex and genetic risk factors. Modifiable risk factors include education, prior history of stroke, lifestyles such as diet, low physical activities, psychological factors like depression, sleep disorders, smoking and alcohol abuse, cardiovascular risk factors and diabetes. AF, atrial fibrillation; BMI, body mass index; VaD, vascular dementia; VCI, vascular cognitive impairment.
The genetic risk factors associated with VaD originated from early studies with rare monogenic stroke disorders like Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy, an inherited SVD that leads to VaD with pathogenic mutations identified on NOTCH3 and HTRA1 genes.10 11 Several genome-wide association studies (GWAS) systematically investigated single-nucleotide polymorphisms associated with VaD. While apolipoprotein E (APOE) is known as a strong risk factor for AD, it also has been associated with VaD. A meta-analysis of 12 cohorts with 2 93 544 subjects including 2935 VaD cases identified the APOE locus as well as 5 other novel loci such as ASTN2 associated with VaD.10 The association of the APOE genetic locus with VaD and VCI was further validated by a recent large-scale GWAS study from 21 cohorts of a total 800 597 participants (including 8702 VaD). In addition, novel genetic loci including SPRY2, FOXA2, AJAP1 and PSMA3 were associated with VaD.12
Modifiable risk factors include education, lifestyle such as physical activities, depression and sleep disorder. A meta-analysis of 19 studies with a total of 404 840 participants found that those who reported high levels of physical inactivity had an increased incidence of all-cause dementia (HR 1.40, 95% CI 1.23 to 1.71) in the 10-year time period before being diagnosed, and a higher risk of developing comorbid cardiometabolic diseases such as diabetes, coronary heart disease, and stroke, 10–15 years before diagnosis of dementia.13 Moreover, in a nationwide Swedish study, among 119 386 subjects at age 50 or older during a mean follow-up period of 10.41 years, 5.5% of subjects with depression vs 2.6% of subjects without depression developed dementia (adjusted OR (aOR) 2.47, 95% CI 2.35 to 2.58; p<0.001), with a stronger correlation found in the incidence of VaD in patients with depression (aOR 2.68, 95% CI 2.44 to 2.95; p<0.001).14 Disordered sleep is associated with a higher risk of dementia as well. A meta-analysis of 18 longitudinal studies with a total of 246 786 subjects found that those with reported sleep disturbances had a higher risk of developing all-cause dementia, AD and VaD.15 It is speculated that lifestyle risk factors such as physical inactivity or sleep disturbance may directly contribute to disease development such as accelerating neurodegeneration and exacerbated inflammatory responses.16 On the other hand, indirect effects on cardiovascular diseases including worsening high blood pressure, dyslipidaemia, obesity and diabetes may contribute to the pathogenesis of VaD.16
Disease mechanisms and pathology
It has been suggested that the pathogenesis of VaD is highly dependent on the nature of vascular pathology that triggers its incidence. Abnormalities in vasculature such as large vessel atherosclerosis, arteriolosclerosis and SVD contribute to both causing and amplifying cerebral and cognitive pathology.1 In terms of ischaemic or haemorrhagic stroke, the magnitude of subsequent tissue loss and affected brain regions are indicative of the pathological processes that arise. The Religious Orders Study and Memory and Aging Project (ROSMAP) studies found that VaD is associated with brain structural alterations, such as multiple microinfarcts (OR 1.22, 95% CI 1.03 to 1.45) and subcortical microinfarcts (OR 1.49, 95% CI 1.20 to 1.84).17 Arteriolosclerosis, in which the walls of small arteries and arterioles are thickened and narrowed, is associated more closely with the damage of the blood–brain barrier (BBB) and glymphatic system dysfunction.18 Increased white matter hyperintensities (WMH) are indicative of neurodegenerative pathology in VaD.8 In a 14-year follow-up study, baseline SVD MRI characteristics such as Fazekas score, WMH volume and percentage of lacunes were significantly higher in VaD than AD patients (p<0.001).19
Cerebral hypoxia and hypoperfusion from these conditions may be the central events underlying the progression of VCI and VaD.20 Subsequently, a cascade of molecular and cellular events is launched including BBB dysfunction, oxidative stress, mitochondrial dysfunction, neuroinflammation and white matter damage. The damage to the BBB and glymphatic system dysfunction result in uncontrolled infiltration of inflammatory factors and other bloodborne molecules into the brain. These changes create a positive feedback loop by further exacerbation of glial reactivity21 and oxidative stress leading to other pathologies.22 Stroke can also cause an increase in the secretion of proteins, proteoglycans, and metalloproteinases into the extracellular space that significantly contributes to further BBB breakdown.23 Activated microglia and astrocytes release cytokines and chemokines that lead to persistent inflammatory activation and excitotoxicity.20 In addition, hypoxia-induced oxidative stress causes a cascade of negative effects such as neurovascular uncoupling and reduced cerebral blood flow (CBF). The increases in reactive oxygen species also disrupt mitochondrial functioning, which further induces hypoxia and oxidative stress.22 The widespread effects of hypoxia on oligodendrocyte damage and white matter demyelination further impair neural transmission and cognitive impairment21 (figure 2).
A cascade of molecular and cellular events underlying the progression of VCI and VaD. Abnormalities in vasculature such as large vessel atherosclerosis, arteriolosclerosis and SVD contribute to both causing and amplifying cerebral and cognitive pathology. In terms of ischaemic or haemorrhagic stroke, the magnitude of subsequent tissue loss and affected brain regions are indicative of the pathological processes that arise. Cerebral hypoxia and hypoperfusion from these conditions may be the central events underlying the progression of VCI and VaD. Subsequently, a cascade of events is launched including BBB dysfunction, oxidative stress, mitochondrial dysfunction, neuroinflammation and white matter damage. The damage to the BBB and glymphatic system dysfunction result in uncontrolled infiltration of inflammatory factors and other bloodborne molecules into the brain. These changes create a positive feedback loop by further exacerbation of glial reactivity and oxidative stress leading to other pathologies. Ultimately, these changes lead to neurotoxicity and cognitive impairment. BBB, blood–brain barrier; SVD, small vessel disease; VaD, vascular dementia; VCI, vascular cognitive impairment.
Biomarker studies
A clinical diagnosis of VaD often involves imaging confirmation of structural abnormalities and clinical assessments of cognition. However, there is a growing interest in biochemical markers to distinguish VaD patients from other dementia patients. A study reported that levels of cerebrospinal fluid (CSF) lipocalin 2 (LCN2), a glycoprotein secreted in response to injury and inflammatory stimuli, were significantly elevated in VaD patients. Changes in CSF LCN2 levels were able to discriminate VaD patients from AD with a sensitivity of 82% and a specificity of 87%, as well as from non-dementia patients with a sensitivity of 78% and a specificity of 82%.24 In a 2019 network meta-analysis comparing neurofilament light (NfL) chain protein expression, they found that there was a significant reduction in CSF NfL levels in MCI subjects compared with AD patients (standard mean difference (SMD) −0.58, 95% CI −1.10 to −0.06), whereas there was a significant increase in CSF NfL levels in VaD subjects compared with AD counterparts (SMD 0.85, 95% CI 0.26 to 1.43).25 Another potential biomarker is the Klotho protein, colloquially known as the anti-ageing protein. Low levels of plasma Klotho were associated with VaD but not with late-onset AD.26 A recent paper categorised candidate biomarker molecules based on VCI pathophysiological processes such as interleukins, CRP, TNFα and others for inflammation, fibrinogen, D-dimer, vWF and others for clotting pathway, sPDGFRβ, MMRs and TIMPs for BBB breakdown, ICAM-1, VCAM-1, P-selection and others for endothelial dysfunction.27
Along with plasma and CSF biomarkers, neuroimaging biomarkers were evaluated as well. Interestingly, cortical and subcortical vascular changes including WMH, lacunes, enlarged perivascular spaces and microbleeds are more specifically associated with VaD.28 In diffuse tensor imaging studies, VaD was mainly linked to widespread white matter damage, particularly in thalamic radiations, while AD exhibited the main damage to parahippocampal pathways.28 In addition, the genu of the corpus callosum was mostly affected in VaD, while the splenium was predominantly affected in AD.29 Moreover, the pattern of fluorodeoxyglucose positron emission tomography in VaD is commonly associated with reduced uptake in deep grey structures, cerebellum, middle temporal gyrus and anterior cingulate, whereas the pattern in AD commonly involves hypometabolism of temporal and parietal lobes.28 Recent advances in arterial spin labelling (ASL) technology, a non-invasive imaging method that detects subtle CBF changes, implicate a role for ASL as a potential predictor of VCI in individuals with high vascular risk factors.30
While there have been exciting advancements in the VaD biomarker field, it should be noted that many of these biomarkers may also change during ageing and in diseases other than VCI/VaD such as systemic diseases. In addition, it is very challenging to distinguish VaD from other neurodegenerative disorders like AD with overlapping heterogeneous pathophysiology. The clinical application of identified fluid biomarkers from research settings is still in question. Future studies will need to develop a panel of biomarkers that better represents the pathophysiology of VCI/VaD and improves clinical distinction of VCI/VaD from normal ageing and other neurodegenerative disorders.
Diagnostic criteria
Diagnosing VaD includes a clinical assessment, cognitive testing, neuroimaging evidence of cerebrovascular lesions with MRI as a gold standard and vascular risk factor profiles.16 The Vascular Impairment of Cognition Classification Consensus Study diagnostic guidelines propose minor and major subdivisions of VCI based on the severity of cognitive impairment and deficits in daily living activities.31 Four subtypes of major VCI are proposed as well, including poststroke dementia, subcortical ischaemic VaD, multi-infarct dementia and mixed dementia of vascular and neurodegeneration.31 32
While clinical subtypes may provide a practical framework for VCI/VaD diagnosis and treatment, genetics and pathophysiological studies imply that VCI subtypes are often interconnected and overlapping at molecular levels, challenging the rigid boundary of clinical classifications. Furthermore, there are high variabilities between vascular burden and cognitive deficits within the spectrum of VCI/VaD. Future work is needed to incorporate not only lesion burden but also the locations of vascular lesions, particularly mapping those strategic locations that result in major cognitive impairment.33 A multimodal comprehensive assessment of vascular injury integrating different metrics into predictive models of VCI development and progression may help address these diagnostic challenges.33
Therapeutics
Symptomatic treatments
Cholinesterase inhibitors (ChEIs) have been extensively studied as a potential treatment for VaD in recent years. In a 54-week study of 885 patients diagnosed with possible or probable VaD, the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-cog) scores in patients who received donepezil for the entire 54 weeks were statistically improved in the first 24 weeks and remained stable in the extension phase.34 In a Cochrane review of 710 patients with VaD, rivastigmine (3–12 mg/day) exhibited a significant advantage in Vascular ADAS, Mini-Mental State Examination (MMSE) and ADAS-Cog measures compared with placebo over a 24-week treatment duration, but not in non-cognitive measures between treatment and placebo.35 Similarly, in a 26-month, double-blind trial of 788 patients with VaD, a significant improvement in the ADAS-cog scale was seen among patients treated with galantamine compared with placebo (−1.8±6.32 in galantamine vs −0.3±5.94 in placebo), but no significant differences in activities of daily living (ADAS-ADL) between the two groups (0.7 vs 1.3; p=0.783).36 In addition, there were negative results from various clinical trials of ChEIs on VaD patients. For example, a meta-analysis of VaD patients suggested that 10 mg/day and 5 mg/day of donepezil treatments showed no significant differences in the MMSE scores compared with placebo.37
Memantine as a partial antagonist of the NMDA receptor shows neuroprotective effects. In a 28-week double-blind trial on 321 VaD patients, memantine (20 mg/day) demonstrated significant improvements in MMSE scores (1.8±3.51 vs 0.2±4.24 in placebo), but no significant difference was observed in the CIBIC-plus scores.38 In a 28-week double-blind trial of 579 participants with VaD, memantine users (20 mg/day) had significant improvements in ADAS-cog (−1.75 points) with no significant differences seen in the Clinical Global Impression of Change (CGI-C).39 In a combined analysis of the two above trials, there were no significant differences between memantine and placebo in clinical global rating and ADL measures, with subgroup analysis suggesting some benefits of memantine in moderate to severe VaD patients.40
The efficacy of dual or combination therapies has been explored as well.41 A meta-analysis of 1647 patients with VaD demonstrated significant improvements in MMSE scores and clinical dementia rating scales favouring a dual therapy with donepezil-nimodipine over monotherapy (OR 2.50, 95% CI 1.92 to 3.09, p<0.00001).42 Similarly, a retrospective study of mixed dementia comparing citicoline plus memantine versus memantine alone demonstrated an increase in the MMSE in the dual treatment, but no significant difference was seen in measures such as ADL, instrumental ADL and neuropsychiatric inventory.43 Moreover, in a 12-month open-label study of 208 subcortical VaD patients, the efficacy of rivastigmine and aspirin combination versus aspirin alone was compared, and it was found that the rate of cognitive decline was statistically higher in the aspirin alone group (combination vs aspirin alone −0.56±0.77 vs −2.71±0.12).44
Overall, there are some reported benefits in symptomatic treatments of VaD, but the extent of benefits is rather limited. Future clinical trials with larger sample sizes are needed to evaluate any potential long-term benefits of combination therapies against VCI and/or VaD progression.
Lifestyle interventions
Non-pharmacological interventions are equally important in dementia prevention and treatment. In a meta-analysis of 736 patients with stroke, both combined aerobic-strength and strength-only interventions exhibited significant benefits on cognition, with combined aerobic-strength training having doubled the benefits. Moreover, training soon after stroke (<3 months) had a greater effect on cognition, while starting exercise even after 3 months still led to a significant cognitive benefit.45 Dietary management is an effective prevention approach for stroke-related dementia development. In a 10-year follow-up of 106 older individuals with a history of stroke, the MIND diet, a hybrid design of the Mediterranean and DASH diets, significantly reduced the decline rate in global cognition, semantic memory and perceptual speed, whereas the Mediterranean or DASH diet alone was not associated with slowing in cognitive decline.46 Smoking cessation is considered to reduce VaD incidence. A 7-year cohort of 46 140 participants showed that long-term quitters (≥4 years) and never-smokers had decreased risk of VaD (HR 0.68, 95% CI 0.48 to 0.96 and HR 0.71, 95% CI 0.54 to 0.95, respectively).47 Management of poststroke behaviour symptoms, including depression and anxiety, can be beneficial. In a meta-analysis of 1091 participants, 8–12 weeks of fluoxetine were associated with MMSE improvements in VaD and AD subjects.48
Multidomain lifestyle modifications are likely more beneficial than a single preventive measure. For example, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability trial with multidomain lifestyle modifications particularly targeting vascular risk modifications showed beneficial effects on executive function and processing speed, especially in older individuals with high cardiovascular risk factors.32 Together, targeting lifestyle modifications shows great promise in early prevention and/or intervention in dementia development in patients with VaD risk factors. Future studies to evaluate the efficacy of combining pharmacological and non-pharmacological interventions in VaD prevention are needed.
Management of comorbid medical conditions
Optimising the management of comorbid medical conditions such as AF, hypertension, hyperlipidaemia and diabetes may reduce the incidence of VCI and VaD. In a 9-year cohort of dementia-free patients, anticoagulant use but not antiplatelet therapy was associated with a 60% lower dementia risk in people with AF.49 On the other hand, studies reported a U-shaped relationship between blood pressure (BP) and dementia. Low BP may deteriorate vascular cognitive decline by inducing cerebral hypoperfusion,50 whereas in a study of 682 poststroke patients, those who had high systolic blood pressure higher than 140 mm Hg had worse Montreal Cognitive Assessment (MoCA) scores 3 and 12 months after an ischaemic event (p<0.0001).51 Therefore, maintaining normotensive BP may be important in preventing the development of VaD.
Long-term use of highly potent and lipophilic statins was associated with reduced dementia risks. In a cohort of 14 807 poststroke patients, statin users had a lower incidence of dementia than non-users. In a dose-response study, for each additional year of statin use, there was a corresponding 20% reduction in dementia risk. A 5 mg increase in a daily dose of statin was linked to an 11% decrease in the risk of dementia.52 Furthermore, two other antidiabetic agents, dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide 1 (GLP-1) agonists may reduce the risks of dementia. In a meta-analysis of diabetic patients, DPP-4 reduced the risk of VaD by 41%.53 In a trial of 8828 patients, long-term injections of dulaglutide, a GLP-1 agonist, attenuated cognitive impairments in diabetic patients. The HR of developing cognitive impairment was reduced by 14% in the dulaglutide users.54
Novel therapeutic interventions
Novel therapeutic strategies for VCI and VaD have been explored. A 2015 clinical trial reported that daily N-acetylcysteine, an antioxidant and anti-inflammatory agent for 6 months improved scores on the Dementia Rating Scale for individuals with MCI.55 Pimavanserin, a serotonin receptor modulator used to treat schizophrenia, was evaluated in phase III clinical trial studies. Among 217 participants (9.7% with VaD), patients treated with pimavanserin had a 13% relapse rate, compared with a 28% relapse in placebo.56 Furthermore, stem cell therapies have been investigated. In a clinical trial of 11 patients with senile VaD, human umbilical cord-derived mesenchymal stem cell infusion for 3 months significantly improved MMSE scores (14.7±2.9 to 18±1.9) and daily life activities (Baseline Barthel Index: 29.6±6.9 to 55.0±4.7).57 In general, stem-cell therapy is an emerging treatment for dementia, with its safety and efficacy to be established with larger clinical trials.
Neuromodulation-based approaches for VaD are another emerging area of research. Non-invasive brain stimulation technologies such as transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) have been investigated. Of note, a prospective pilot study assigned 10 patients with poststroke cognitive impairment to high-frequency rTMS of the ipsilateral dorsolateral prefrontal cortex (DLPFC) for a total of 10 sessions. Cognitive performance was improved following completion of rTMS 2 and 14 weeks follow-up. The cytokine levels were reduced after rTMS, with interleukin-6 levels in particular inversely correlated with the improvements in the auditory verbal learning test (r=0.928).58 Moreover, a recent meta-analysis of 10 rTMS trials of PSCI with 347 participants in total showed an improvement in MoCA scores in the rTMS group when targeting the DLPFC (SMD 0.54, 95% CI 0.31 to 0.76, p<0.00001).59 Furthermore, a meta-analysis of 8 human trial studies testing the effect of tDCS on cognitive performance indicated moderate heterogeneity (I2=50.43%), and an overall effect size of 0.26 favouring treatment (95% CI −0.03 to 0.55, p=0.077).60
Despite considerable progress, challenges remain regarding the implementation of these novel therapies. Many neuromodulation-based therapies are still considered experimental, and therefore, approvals from regulatory agencies can lag. Moreover, logistical challenges persist such as a lack of clinical infrastructure and associated workforce that can deliver neuromodulation therapies. From an ethical standpoint, neuromodulation-based therapies are emerging technologies with uncertain risks. The consent processes in vulnerable patient populations with cognitive impairment require special attention.
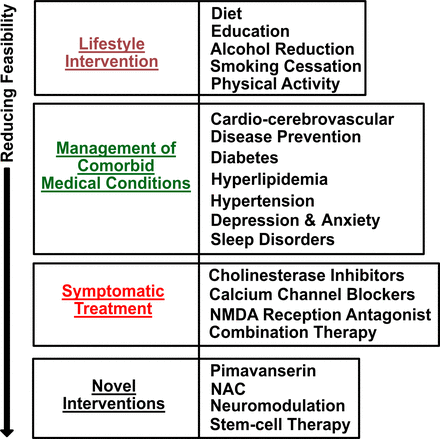
In summary, studies of novel therapeutic interventions for VCI and/or VaD (figure 3) show great promise. Future research is needed regarding the determination of optimal doses, duration of interventions, patient selection, as well as study replication in multicentred clinical trials. More importantly, there is a pressing need to develop new therapeutics and interventions targeted on VaD-specific pathways and disease mechanisms.
A summary of therapeutic interventions for VCI and VaD. The therapeutic interventions including lifestyle interventions, management of comorbid medical conditions, symptomatic treatment as well as novel interventions are ranked for the feasibilities in treating VCI and/or VaD based on available data. VaD, vascular dementia; VCI, vascular cognitive impairment.
Summary
Here, we reviewed the risk factors, disease mechanisms, biomarkers, diagnostic criteria and therapeutics for VaD. Given the heterogeneity of VaD, risk factors are vast. Defining the disease mechanisms responsible for VaD is an ongoing challenge, and biomarkers for VaD are an area of growing interest with promises for risk stratification and patient selection for early intervention. Lastly, therapeutic options remain limited with mostly symptomatic treatments. Lifestyle modification is still the most economical and accessible intervention. Neuromodulation and stem-cell-based therapies could represent the future direction of VaD interventions. Much research is needed to advance the understanding of VaD pathogenesis, which could guide future development of novel diagnostic biomarkers and therapeutic interventions targeted at VCI and/or VaD.
Ethics statements
Patient consent for publication
Footnotes
SN, AH and PH are joint first authors.
SN, AH and PH contributed equally.
Contributors SN, AH, PH, SI, JC, WF and DC conducted literature search, review, analyses and figure preparation, scientific discussions as well as wrote the paper. DC is responsible for the overall content as the guarantor.
Funding This work was funded by U.S. Department of Veterans Affairs (I01BX003380, I01BX005934) U.S. National Institute of Health (RO1 AG048923, RO1 AG068030, RO1 AG074010, UH2 AG083258)
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.