Abstract
Background and purpose Symptomatic internal carotid artery stenosis (sCAS) is an essential cause of transient ischaemic attack (TIA) or minor stroke. We aimed to evaluate whether the superiority of aspirin-ticagrelor over aspirin-clopidogrel varies between patients with sCAS or not.
Methods This was a post-hoc analysis of the High-Risk Patients with Acute Nondisabling Cerebrovascular Events-II (CHANCE-2) trial, all of which were CYP2C19 loss-of-function alleles carriers. The primary exposures of interest were the treatment group and sCAS status. The primary efficacy endpoint was the new stroke assessed within 90 days.
Results A total of 5920 (92.3%) from 6412 were analysed, including 197 (3.3%) with sCAS and 5723 (96.7%) without sCAS. Stroke recurrence occurred in 13 (12.15%) and 11 (12.22%) patients with sCAS who received aspirin-ticagrelor and aspirin-clopidogrel, respectively (adjusted HR, 1.04; 95% CI, 0.46 to 2.36; p=0.930). Among patients without sCAS, there were 158 cases (5.52%) of new strokes in the aspirin-ticagrelor group and 222 cases (7.76%) in the aspirin-clopidogrel group (HR, 0.70; 95% CI, 0.57 to 0.86; p=0.0006). The treatment-by-sCAS subtype was not significant (p=0.405).
Conclusions Genotype-guided dual antiplatelet treatment with aspirin-ticagrelor may be beneficial for preventing recurrent strokes in patients without sCAS; however, it appears less effective in those with sCAS. No significant interaction was found between the treatment and sCAS subtypes.
Trial registration number NCT04078737.
WHAT IS ALREADY KNOWN ON THIS TOPIC
The superiority of aspirin-ticagrelor over aspirin-clopidogrel has already been explored in high-risk transient ischaemic attack (TIA) or minor stroke (MS) patients. However, the consistency of the above benefit was not evaluated between participants with symptomatic internal carotid artery stenosis (sCAS) or not.
WHAT THIS STUDY ADDS
This study found that aspirin-ticagrelor was more effective than aspirin-clopidogrel in decreasing the risk of recurrent stroke within 90 days in patients without sCAS, but this benefit was not observed in those with sCAS.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Caution is advised when evaluating the effectiveness of dual antiplatelet therapy in patients with high-risk TIA or MS with sCAS. A prospective and well-designed study is necessary for a more thorough evaluation.
Introduction
Symptomatic internal carotid artery stenosis (sCAS) is a significant cause of transient ischaemic attack (TIA) or minor ischaemic stroke (MIS). Patients with sCAS face an increased risk of early stroke recurrence, ranging from 2.7% within the first day of symptom onset to 18.8% within 90 days.1 2 Over the past decade, there has been a substantial decline in new stroke events for patients with TIA/MIS due to the addition of clopidogrel to aspirin.3–5 Randomised controlled trials have shown that genotype-guided aspirin-ticagrelor is superior to aspirin-clopidogrel in reducing stroke recurrence in patients with high-risk TIA or MIS who are carriers of CYP2C19 loss-of-function (LOF).6 7
A post hoc analysis from the POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischaemic Stroke) trial indicated that the risk of ischaemic stroke events was increased in patients with sCAS. Meanwhile, the effect of aspirin-clopidogrel was not significantly different in patients with and without arterial stenosis.8 However, another exploratory study of THALES (The Acute Stroke or Transient Ischaemic Attack Treated With Ticagrelor and ASA for Prevention of Stroke and Death) trial showed dual antiplatelet therapy with aspirin and ticagrelor was more effective in symptomatic large artery atherosclerosis.9 However, both analyses were restricted to comparing the addition of ticagrelor or clopidogrel to aspirin against aspirin monotherapy. Recently, the Roundtable of Academia and Industry for Stroke Prevention Conference emphasised the significant uncertainties in the existing evidence base regarding the rational intervention of sCAS.10 The CHANCE-2 (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events-II) trial indicated that aspirin-ticagrelor was modestly more effective than aspirin-clopidogrel in reducing the occurrence of new strokes among MIS/TIA patients who are carriers of CYP2C19 LOF alleles. However, it remains unclear whether genotype-guided treatment with aspirin-ticagrelor offers additional benefits for high-risk patients with sCAS. In this post-hoc study of the CHANCE-2 trial, we aimed to compare the efficacy and safety of two dual antiplatelet therapies—aspirin-ticagrelor vs aspirin-clopidogrel—in patients with and without sCAS.
Methods
Study design and participants
The data of this study were derived from the CHANCE-2 trial, which focused on high-risk TIA or MIS patients with CYP2C19 LOF alleles. A detailed description of the rationale, design and methods of the CHANCE-2 trial is publicly available.11 This was a randomised, multicentre, double-blind, placebo-controlled study conducted across 202 sites in mainland China. A total of 6412 participants were enrolled and randomised in a 1:1 ratio to receive either ticagrelor added to aspirin or clopidogrel plus aspirin. Patients in both groups received a loading dose (75 to 300 mg) of aspirin, followed by 75 mg/day for 21 days. In the aspirin-ticagrelor group, patients received a loading dose (180 mg) of ticagrelor on day 1, followed by a daily dose of 90 mg twice from days 2 to 90, along with a placebo for clopidogrel. In the aspirin-clopidogrel group, patients received a loading dose (300 mg) of clopidogrel on day 1, followed by a daily dose of 75 mg from days 2 to 90, along with a placebo for ticagrelor twice daily. In this report, we derived patients who completed at least one carotid imaging: digital subtraction angiography, magnetic resonance imaging (including MRA), CT scan (including CTA) or carotid ultrasound. The investigators from each site obtained written informed consent from all participants or their legal guardians prior to their enrolment in the study.
Data collection and assessment of sCAS
Through face-to-face interviews, the relevant information about demographic attributes, medical history, cardiovascular risk factors, physical assessments, laboratory examinations, medical interventions and evaluations from the pre-stroke modified Rankin Scale (mRS) score, NIHSS and ABCD2 were obtained by trained neurologists at each site. This was done following a standard procedure for data collection established by the steering committee of the CHANCE-2 trial. The definition of sCAS was ≥50% stenosis in the extracranial cervical internal carotid artery, which led to the following symptoms: amaurosis fugax, TIA or ischaemic stroke ipsilateral to the lesion.
In this study, we permitted conventional angiography measurements to substitute for all other types of assessments, while MRA or CTA measurements could be used as alternatives to carotid ultrasound. We conducted a central analysis of infarct territory on brain imaging.
Outcome measurement
The primary endpoint was the occurrence of a new stroke, which included both ischaemic and haemorrhagic strokes, within 90 days. Secondary endpoints comprised the following: the incidence of new strokes within 30 days; the occurrence of any vascular event, defined as a composite of stroke, TIA, myocardial infarction and vascular death; the rate of new ischaemic strokes within 90 days; and the incidence of disabling strokes (defined as an mRS score of ≥2) within 90 days. Additionally, the primary safety endpoint was assessed as moderate/severe bleeding events, defined as the GUSTO (Global Utilisation of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) criteria, occurring within 90-day (see online supplemental table S1).12
Supplementary data
Statistical analysis
Continuous variables were reported as medians with IQRs and categorical variables as counts with percentages (%). For baseline characteristics, the differences were evaluated first between the intervention group (aspirin-ticagrelor) and the control group (aspirin-clopidogrel), further considering the presence of sCAS. For continuous and categorical variables, the Kruskal–Wallis and X2 tests were used, respectively. For the cumulative incidence of the new strokes, Kaplan–Meier curves were shown in each group for 90 days. Differences in study endpoints during this follow-up were analysed by the Cox proportional hazards regression model. This model incorporated study centres as a random effect and reported HRs along with 95% CIs. In cases with multiple events of the same type, only the time to the first event was included in the analysis. Data from patients who did not experience any events during the study were censored either at the termination of the trial or at the time of non-vascular-related death. The proportional hazard assumption for each model was evaluated by testing the interaction between treatment and time. To assess whether the effect of the intervention on clinical endpoints was influenced by the presence of sCAS, we tested the interaction between intervention and sCAS status in the Cox model for the included population. The Cox model incorporated an interaction between time and treatment to evaluate the assumption of proportional hazards.
Data were analysed by SAS software (version 9.4; SAS Institute). All statistical tests were two-tailed, and a significance level of p<0.05 was established for all analyses.
Results
Participants
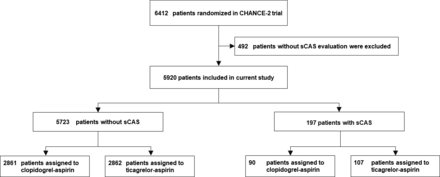
Between 23 September 2019 and 22 March 2021, a total of 6412 patients with acute minor stroke or high-risk TIA were randomised and enrolled in the CHANCE-2 trial. Among these, 492 patients (7.67%) lacked vascular imaging results. Consequently, 5920 patients (92.33%) were included in the study (figure 1). The characteristics of both included and excluded patients are summarised in online supplemental table S2. Compared with the excluded patients, the proportion of Han ethnicity in the included subgroup was slightly higher. Among the 5920 patients included in the study, the median age was 64.83 years (IQR, 56.95–71.38), with 2004 (39%) identified as female. Of these, 197 patients were diagnosed with sCAS, while the remaining 5723 patients did not have this condition. As presented in table 1, of the 197 patients with sCAS, 39 (19.8%) were female, and the median age was 68.66 years (IQR, 61.79–74.56). In contrast, among the 5,723 patients without sCAS, 1965 (33.34%) were female, and the median age was 64.70 years (IQR, 56.86–71.22). Patients with and without sCAS in the aspirin-ticagrelor and aspirin-clopidogrel groups were well-balanced regarding the diagnostic investigation methods (online supplemental table S3). Patients with sCAS tended to be older, exhibited lower diastolic blood pressure and had a higher prevalence of a medical history involving previous ischaemic strokes and myocardial infarctions. Additionally, these patients were more likely to have experienced a TIA, have a history of previous antiplatelet and lipid-lowering therapy and be classified as having intracranial artery stenosis (ICA) or symptomatic intracranial artery stenosis (sICA). Table 2 presents the demographic and clinical characteristics of patients with and without sCAS, as well as those receiving different treatments. Notably, the aspirin-ticagrelor and aspirin-clopidogrel groups demonstrated a well-balanced distribution across both groups.
Study flow diagram for ticagrelor vs clopidogrel treatment in patients with or without sCAS groups. sCAS, symptomatic cranial artery stenosis.
Baseline characteristics of patients with/without sCAS
Baseline characteristics of patients receiving different treatments with/without symptomatic internal carotid artery stenosis
Efficacy outcomes
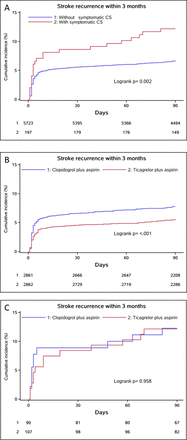
As shown in online supplemental table S4, the rate of recurrent stroke in this study population was 6.82% (404 patients) at the 3 month mark. Specifically, the risk of recurrent stroke was 6.64% in patients with ICAS and 12.18% in those without ICAS at the 3 month follow-up. Patients with sCAS had a higher stroke recurrence risk during 90 days (12.18% vs 6.64%; HR, 1.88; 95% CI, 1.24 to 2.84; p=0.003). After adjusting for age, sex, medical history (including hypertension, diabetes mellitus, previous ischaemic stroke and previous TIA, previous antiplatelet therapy), smoking status, duration from symptom onset to randomisation, intermediate/poor CYP2C19 LOF allele carrier, qualifying event and prior lipid-lowering therapy, the risk of recurrent stroke remained elevated for sCAS group. The adjusted HR was 1.82 (95% CI, 1.20 to 2.76; p=0.005).
In the sCAS group, 13 patients (12.15%) receiving aspirin-ticagrelor and 11 patients (12.11%) receiving aspirin-clopidogrel had the primary efficacy outcome of recurrent stroke within 90 days. In contrast, among patients without sCAS, r 158 patients (5.52%) receiving aspirin-ticagrelor and 222 patients (7.76%) receiving aspirin-clopidogrel occurred recurrent stroke. Aspirin-ticagrelor was associated with a lower rate of recurrent stroke compared with aspirin-clopidogrel in the sCAS group (HR, 0.70; 95% CI, 0.57 to 0.86; P < 0.001) but not in sCAS group (HR, 1.04; 95% CI, 0.46 to 2.36; P = 0.930). The treatment-by-sCAS subtype interaction was nonsignificant (interaction p=0.405) (see online supplemental table S5 and figure 2). For secondary efficacy outcomes, including strokes occurring within 30 days, and for combined vascular events and ischaemic strokes within the 90-day follow-up, similar results were observed. For disabling strokes within 90 days, no significant differences were found between aspirin-ticagrelor and aspirin-clopidogrel in both group patients with or without sCAS (see online supplemental table S5 and figure S1).
Kaplan–Meier plots of new stroke in patients with and without sCAS or different treatments. sCAS, symptomatic cranial artery stenosis; new stroke in all populations with or without sCAS (A) and new stroke in the aspirin-ticagrelor and aspirin-clopidogrel groups in patients without sCAS (B), and with sCAS (C).
Safety outcomes
The aspirin-clopidogrel and aspirin-ticagrelor groups experienced comparable rates of primary safety endpoint of severe or moderate bleeding, regardless of the sCAS status. In the sCAS population, the rates were 1.84% for ticagrelor-aspirin and 0% for aspirin-clopidogrel, while in the population without sCAS, the rates were 0.24% for aspirin-ticagrelor and 0.47% for aspirin-clopidogrel (see online supplemental table S5). For the secondary safety endpoint of any bleeding, compared with aspirin-clopidogrel, a higher risk was observed only in those receiving aspirin-ticagrelor in the sCAS-free population (5.52% vs 2.66%; HR, 2.11; 95% CI, 1.60 to 2.77; P<0.001); however, the interaction effect was nonsignificant (p=0.973 for interaction). Moreover, no significant differences were observed in intracranial haemorrhage or mortality between the two groups in patients with or without sCAS.
Discussion
This is a post-hoc study of the CHANCE-2 trial, which evaluated the efficacy and safety of aspirin-ticagrelor compared with aspirin-clopidogrel stratified by the status of sCAS. We found that sCAS was associated with a significantly increased risk of recurrent stroke. Aspirin-ticagrelor was superior to aspirin-clopidogrel in reducing the risk of recurrent stroke within 90 days in patients without sCAS, but this superiority was not observed in those with sCAS. However, there was no significant difference in the effect of dual antiplatelet therapy with aspirin-ticagrelor or aspirin-clopidogrel between patients with and without sCAS. Furthermore, the risk of severe or moderate bleeding events was similar in both treatment groups in patients with or without sCAS.
The proportion of patients with sCAS in the CHANCE-2 trial (3.3%) was lower than that in the UK-TIA trial (6%) but higher than that in the POINT trial (1.7%).8 13 14 However, it should be noted that carotid imaging in the UK-TIA trial was only performed in patients suspected with large-artery disease. The difference between our study and the POINT trial may be attributed to racial differences or CYP2C19 metabolizser status, but further studies are needed to clarify this issue. Another study focusing on recent TIA or ischaemic stroke in the internal carotid artery territory reported a higher prevalence of sCAS stenosis (12.5%), possibly due to their inclusion of patients with anterior circulation infarction. In general, there needs to be more consistent data on the prevalence of sCAS, and more clinical research is required to address this gap.
In our analysis, the presence of sCAS was connected to an elevated risk of recurrent stroke in patients with CYP2C19 LOF alleles, consistent with multiple previous studies, which have shown that noncardioembolic stroke patients with the ipsilateral atherosclerotic disease face a significantly elevated absolute risk compared with other subtypes of stroke.3 4 15–18 Our analysis emphasise the increased risk of stroke recurrence in patients with sCAS, despite receiving aspirin-ticagrelor. This underscores the importance of early screening for sCAS in patients with CYP2C19 LOF alleles and implementing additional secondary prevention strategies. According to current evidence, patients with sCAS should receive intensive medical management. This includes implementing dual antiplatelet therapy with aspirin and clopidogrel for the initial 3 months following an ischaemic event. The 2021 AHA/ASA guidelines recommend intensive medical therapy for reducing stroke risk in TIA or stroke patients with carotid artery stenosis. This comprehensive approach includes antiplatelet therapy, lipid-lowering treatment and management of hypertension to reduce the risk of subsequent strokes effectively.19 In patients with sCAS who have anatomical or medical conditions that elevate surgical risks—such as radiation-induced stenosis or restenosis following carotid endarterectomy (CEA)—carotid artery stenting is a reasonable alternative to minimise the rate of periprocedural complications. Previous studies have also demonstrated the benefits of surgery in specific subgroups of sCAS. Both published in 1991, the North American Symptomatic Carotid Endarterectomy trial20 21 and the European Carotid Surgery Trial,22 23 demonstrated the advantages of surgery in specific subgroups of symptomatic patients. However, limited data has compared CEA with optimal medical therapy for those with symptomatic carotid stenosis. Furthermore, CEA has also been evaluated compare to carotid artery stenting (CAS).
Several clinical researches have investigated the impact of ICA on the prognosis of cerebrovascular events. As reported in a subgroup study of the CHANCE-2 trial, ticagrelor therapy reduced the stroke recurrence rate in patients with ICA.24 A randomised controlled trial included patients with acute large-vessel ischaemic stroke and found that the ticagrelor group had better clinical outcomes compared with the clopidogrel group.25 As indicated in this post-hoc study, intensive antiplatelet therapy with aspirin-ticagrelor did not reduce the risk of stroke in patients with sCAS. While intracranial and extracranial artery stenoses are frequently observed in patients with ischaemic strokes, they represent distinct phenotypes of systemic atherosclerosis and may require different treatment approaches. The finding from this study could be partly explained by the profile of patients who received aspirin-ticagrelor or aspirin-clopidogrel. The absolute rate of patients with ICA and sICA was higher in the aspirin-ticagrelor group than the aspirin-clopidogrel group. It is essential to note that the smaller sample size of sCAS results in a higher margin of error or a lower confidence level. This trend may be attributed in part to statistical factors. However, a subgroup analysis of the CSPS.com trial found that adding cilostazol to aspirin or clopidogrel did not have superiority in preventing vascular events, including recurrent stroke in patients with extracranial arterial stenosis.26 The potential biological mechanisms underlying the observed lack of treatment-by-sCAS interaction might be attributed to the ceiling effect in antiplatelet therapy for patients with sCAS. As no additional benefits were found with intensive antiplatelet therapies, carotid revascularisation may produce promising outcomes in this subgroup of patients. In addition to revascularisation procedures when necessary, promising therapeutic approaches, including anti-inflammatory drugs, novel lipid-lowering agents and direct oral anticoagulants, need further investigation.
Limitations
This analysis has several limitations. First, the application of our findings might only fit Asian patients because the CHANCE-2 trial mainly enrolled Chinese patients. Second, it is essential to note that only a minority of participants in this study had sCAS. As a post-hoc analysis with a small sample size, the results should be interpreted with caution, as they may be misleading and should not be regarded as conclusive evidence. Finally, all participants in this study were CYP2C19 LOF alleles carriers, and it remains uncertain whether the findings can be generalised to non-carriers. While there are currently no specific guidelines for antiplatelet therapy in this population, the findings of this study provide valuable insights. Nevertheless, a more comprehensive assessment requires the conduction of a well-designed study specifically focusing on stroke patients with sCAS.
Conclusions
In this post-hoc analysis of the CHANCE-2 trial, patients with sCAS exhibited a significantly higher risk of new stroke than those without sCAS among individuals with CYP2C19 LOF alleles. Genotype-guided dual antiplatelet therapy involving aspirin and ticagrelor may offer advantages in preventing recurrent strokes in patients without sCAS; however, it may not be effective for those with sCAS, despite the absence of a treatment-by-sCAS subtype interaction.
Data availability statement
Data are available upon reasonable request. Requests for access to the data reported in this paper will be considered by the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the ethics committee of Beijing Tiantan Hospital (IRB approval number: KY2019-035-02) and all participating centers. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
Both corresponding authors had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. We thank all study participants, their relatives and the members of the survey teams at the 202 centres of the CHANCE-2 study.
Footnotes
X @yilong
XX and JJ contributed equally.
Contributors XX, JJ, XM and YW contributed to the conception and design of the study; AW, QX, HL and YJ contributed to the acquisition and analysis of data; XX and JJ contributed to drafting the text or preparing the figures. XZ, JL, PC and YW contributed to revising a draft of this manuscript. Corresponding authors had full access to all the data in the study, acted as guarantors for the study and had final responsibility for the decision to submit for publication. All authors vouch for the completeness and accuracy of the data and for the fidelity of the study. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding This study was supported by grants from the National Natural Science Foundation of China (U20A20358, 82471356). CAMS Innovation Fund for Medical Sciences (2019-2M-5-029).
Disclaimer The funder of the study was not responsible for the study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.