Abstract
Background The efficacy of percutaneous transluminal angioplasty and stenting (PTAS) relative to medical management in treating symptomatic intracranial arterial stenosis (ICAS) varies based on the qualifying artery. This study aims to evaluate PTAS compared with medical therapy alone in cases of ICAS involving the internal carotid artery (ICA), middle cerebral artery (MCA), vertebral artery (VA) and basilar artery (BA).
Methods This study involves a thorough pooled analysis of individual patient data from two randomised controlled trials, evaluating the efficacy of PTAS in comparison to medical management for symptomatic ICAS with different qualifying arteries. The primary outcome was stroke or death within 30 days postenrolment, or stroke in the region of the qualifying artery beyond 30 days through 1 year. A methodology based on intention-to-treat was employed, and HR accompanied by 95% CIs were used to convey risk estimates.
Results The data of 809 individuals were collected from Stenting vs Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis trial and China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis trial. Four hundred were designated for PTAS, while 409 were assigned to medical therapy alone. For the primary outcome, patients with symptomatic BA stenosis had a significantly higher risk of receiving PTAS compared with medical therapy (17.17% vs 7.77%; 9.40; HR, 2.38 (1.03 to 5.52); p=0.04). However, PTAS had no significant difference in patients with symptomatic ICA (26.67% vs 16.67%; HR, 1.68 (0.78 to 3.62); p=0.19), MCA (8.28% vs 9.79%; HR, 0.85 (0.42 to 1.74); p=0.66) and VA stenosis (9.52% vs 10.71%; HR, 0.91 (0.32 to 2.62); p=0.86) compared with medical therapy.
Conclusions PTAS significantly increases the risk of both short-term and long-term stroke in patients with symptomatic BA stenosis. Without significant technological advancements to mitigate these risks, PTAS offers limited benefits. For symptomatic ICA, MCA and VA stenosis, PTAS provided no significant advantage.
WHAT IS ALREADY KNOWN ON THIS TOPIC
The influence of specific qualifying arteries on percutaneous transluminal angioplasty and stenting (PTAS) or medical management outcomes in intracranial arterial stenosis (ICAS) cases is insufficiently explored and requires additional elucidation.
WHAT THIS STUDY ADDS
Our findings reveal that PTAS considerably increases the risk for patients with symptomatic basilar artery (BA) stenosis compared with medical therapy alone. Conversely, no significant benefits were observed with PTAS in cases involving symptomatic ICA, middle cerebral artery (MCA) and VA stenosis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
PTAS is unlikely to offer substantial benefits for symptomatic BA stenosis patients, unless technological advancements significantly reduce stroke risk. PTAS showed no significant benefits in symptomatic ICA, MCA and VA stenosis, and further trials are needed
Introduction
Intracranial arterial stenosis (ICAS) is a major contributor to ischaemic stroke and presents a significant challenge to global health.1 2 Studies show that individuals with ICAS, particularly those experiencing significant stenosis,3 4 face a heightened risk of recurrent events and negative outcomes. PTAS is gaining popularity among physicians as a therapeutic option.5–8 However, results from the Stenting vs Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial and the Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT) trial indicate that PTAS presents an increased risk of both short-term and long-term stroke or mortality in comparison to medical therapy.9 10 Additionally, findings from the China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS) trial demonstrate no notable variation in the risks of stroke or mortality within the initial 30 days, nor in the occurrence of stroke in the impacted arterial regions from 30 days to 1 year when compared with medical therapy alone.11 The current body of research has not yet definitively established the benefits of PTAS for individuals experiencing symptomatic ICAS. The efficacy of these treatment approaches in particular patient subpopulations is still inadequately investigated and warrants additional study. In order to fill this gap, we have conducted a thorough examination of aggregated raw data from multicentre randomised controlled trials to evaluate the effectiveness of PTAS in comparison to medical therapy alone in patients experiencing symptomatic ICAS.12
The anatomical characteristics and physiopathology of different qualifying arteries vary considerably,13 14 potentially leading to variations in PTAS effectiveness. However, the precise impacts of PTAS or medical therapy alone on these varied arterial structures have yet to be defined. Consequently, it is essential to examine the outcomes of these treatment options in patients with symptomatic ICAS, segmented by different qualifying arteries, to refine clinical decision-making and enhance patient results.
To this end, we initiated a collaboration between the Stenting vs Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) and China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS) studies to evaluate the impacts of PTAS and medical therapy alone on symptomatic ICAS patients with various qualifying arteries, leveraging individual patient data aggregated from both trials.
Methods
Study design and population
Through 1 January 2024, we combed through databases such as PubMed, MEDLINE, EMBASE, the Cochrane Library and ClinicalTrials.gov in search of randomised controlled studies that compared stenting to medical care with multiple centres. The following trials were found: VISSIT,10 SAMPPRIS9 and CASSISS.11 Unfortunately, access to data from the VISSIT trial was not permitted by the principal investigator. Consequently, we relied only on the SAMMPRIS and CASSISS studies to acquire individual patient data from their respective investigators.
Patient and public involvement
Using data from the SAMMPRIS and CASSISS trials, a pooled post hoc analysis was conducted at the patient level. These studies previously revealed that patients with symptomatic ICAS were compared with those receiving medical therapy alone to those receiving PTAS.9 11 The individuals who took part in the study gave their written consent after suffering a recent transient ischaemic attack (TIA) or stroke. All necessary ethical review boards gave their stamp of approval to the research plans. Both SAMMPRIS and CASSISS have recruitment periods; the former ran from 2008 to 2011 and the latter from 2014 to 2016.
Outcomes
The composite primary outcome was stroke or death within 30 days after enrolment, or stroke in territory of qualifying artery beyond 30 days through 1 year. Stroke within the same territory within 2 and 3 years, death within 3 years, any stroke within 3 years, myocardial infarction within 3 years and a debilitating stroke or death within 3 years were all considered secondary outcomes.
Data analysis
An intention-to-treat approach was used across all studies. Data from each trial were cleansed and harmonised to facilitate combined analysis. χ2 tests analysed categorical variables, presented as counts (proportions). The Kolmogorov-Smirnov test assessed the normality of continuous variables. Continuous variables with normal distributions were subjected to independent samples t-tests and presented as mean±SD. The Mann-Whitney U test was used to evaluate non-normally distributed continuous variables, which were represented by median values (25th–75th percentile).
We used trial-specific Cox proportional hazard regression models to compare PTAS and medical therapy groups’ outcomes, and we estimated HR and 95% CI based on the qualifying arteries. Starting on the date of randomisation, the duration of the follow-up was determined until the first incident of any kind, including death, withdrawal or loss to follow-up. The log-rank test compared the total number of occurrences between the groups, whereas Kaplan-Meier survival analysis produced time-to-event curves.
Subgroup analyses for the primary outcome include age, sex, race, body mass index (categories: <24; 24–28; >28), hypertension, diabetes mellitus, hyperlipidaemia, antithrombotic therapy, type of qualifying event (TIA or stroke), timing of the latest ischaemic event relative to randomisation (<30 days; 30–60 days; >60 days) and degree of stenosis (70%–79%; 80%–89%; 90%–99%). The interaction effects between interventions and these factors were evaluated through the application of generalised linear regression models, utilising a binomial distribution and a log link function. Statistical significance was assessed using a two-sided p<0.5. All analyses were performed utilising R, V.3.4.4.
Results
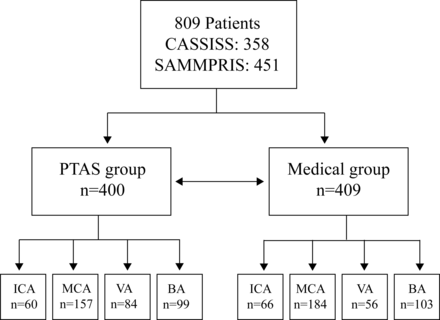
We analysed data from 809 individuals, including 451 from SAMMPRIS and 358 from CASSISS. Of these, 400 (224 from SAMMPRIS and 176 from CASSISS) were assigned to PTAS, and 409 (227 from SAMMPRIS and 182 from CASSISS) to medical therapy alone (figure 1). There were 58.69 years of age on average, with 66.13% of the participants being men. Asians made up 45.48% of the patients, while white people made up 39.80%. In terms of baseline characteristics, the medical and PTAS groups were rather evenly distributed (table 1).
Study profile. BA, basilar artery; CASSISS, China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis; ICA, internal carotid artery; MCA, middle cerebral artery; PTAS, percutaneous transluminal angioplasty and stenting; SAMMPRIS, Stenting vs Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis; VA, vertebral artery.
Baseline characteristics of study participants
Clinical outcomes by qualifying artery
Clinical outcomes for different symptomatic qualifying arteries were compared between the PTAS and medical groups. In patients with basilar artery (BA) stenosis, PTAS was associated with a significantly higher risk of the primary outcome (17 (17.17%) vs 8 (7.77%); HR, 2.38 (1.03 to 5.52); p=0.04) (table 2); stroke in the same territory within 2 year (17 (17.17%) vs 8 (7.77%); HR, 2.39 (1.03 to 5.55); p=0.04) (table 2) and stroke in the same territory within 3 year (18 (18.18%) vs 8 (7.77%); HR, 2.55 (1.10 to 5.84); p=0.03) (table 2). No significant differences were observed between PTAS and medical therapy in patients with internal carotid artery (ICA) stenosis (16 (26.67%) vs 11 (16.67%); HR, 1.68 (0.78 to 3.62); p=0.19), middle cerebral artery (MCA) stenosis (13 (8.28%) vs 18 (9.79%); HR, 0.85 (0.42 to 1.74); p=0.66), and vertebral artery (VA) stenosis (8 (9.52%) vs 6 (10.71%); HR, 0.91 (0.32 to 2.62); p=0.86) (table 2).
Primary and secondary outcomes in different stenosis locations
Subgroup analysis of BA stenosis
Subgroup analysis of symptomatic BA stenosis revealed higher risks associated with PTAS in patients who were white (HR, 4.24 (1.17 to 15.41)), had diabetes mellitus (HR, 3.30 (1.05 to 10.38)), hyperlipidaemia (HR, 3.50 (1.13 to 10.87)), experienced a TIA (HR, 10.74 (1.37 to 83.91)) or had their latest ischaemic event to randomisation within 30 days (HR, 3.72 (1.21 to 11.42)) (figure 2).
Subgroup analysis for the primary outcome of BA stenosis. BA, basilar artery; PTAS, percutaneous transluminal angioplasty and stenting; TIA, transient ischaemic attack.
Comparison of primary outcomes across different qualifying arteries
In the PTAS group, there were no significant differences in primary outcomes between the anterior and posterior circulations (13.36% vs 13.66%; HR, 1.04 (0.61 to 1.78); p=0.87) (figure 3A, table 3). However, notable differences were observed across different qualifying arteries (ICA (26.67%) vs BA (17.17%) vs MCA (8.28%) vs VA (4.76%); p=0.003) (figure 3B, table 3).
Kaplan-Meier curves for the cumulative probability of the primary outcome in different qualifying arteries. The shading indicates 95%CI of the primary outcome. BA, basilar artery; ICA, internal carotid artery; MCA, middle cerebral artery; PTAS, percutaneous transluminal angioplasty and stenting; VA, vertebral artery.
Comparison of the primary outcome of different qualified arteries
In the medical group, the differences between anterior and posterior circulations were not statistically significant (11.60% vs 8.81%; HR, 0.75 (0.40 to 1.38); p=0.37) (figure 3C, table 3), and similar results were seen across the various qualifying arteries (figure 3D, table 3).
Discussion
This post hoc analysis using data from the CASSISS and SAMMPRIS studies suggests that PTAS notably increases the risk of both short-term and long-term stroke in patients with symptomatic BA stenosis. Subgroup analysis indicates that PTAS poses a higher risk for patients who are white, hypertensive, diabetic, have hyperlipidaemia, experienced a TIA or had an ischaemic event within 30 days prior to randomisation, compared with medical therapy. While PTAS resulted in higher rates of primary outcomes in patients with ICA stenosis, it did not show statistically significant differences for those with MCA and VA stenosis, where rates were actually lower compared with medical therapy. Additionally, PTAS resulted in significant variations in primary outcomes across different qualifying arteries.
Our study suggests that patients with symptomatic BA stenosis who underwent PTAS experienced an elevated risk of stroke or death compared with those receiving medical therapy, possibly due to disruption of blood flow in perforated vessels.15 In our examination of potential causes, a subgroup analysis was performed on patients with BA stenosis. This analysis indicated that PTAS was associated with a higher risk compared with medical therapy for the primary outcome among patients who are white, or have hypertension, diabetes mellitus, hyperlipidaemia, TIA or experienced an ischaemic event less than 30 days prior to randomisation. The elevated risk observed in white patients may stem from anatomical differences across races.16 The CASSISS investigation excluded individuals with isolated perforator strokes without arterioarterial embolisation or distal perfusion deficits and mostly included Asian participants.11 15 There was a higher risk associated with PTAS compared with medical therapy in cases when BA stenosis and TIA were the qualifying events. The WASID,17 SAMMPRIS9 and CASSISS11 studies all showed that TIA patients had extremely low risks of stroke and death after medical therapy, so it is reasonable to assume that this group is low risk and will not benefit from PTAS. Based on these results, it seems like it would be too soon to evaluate the effectiveness of PTAS with medical therapy alone for patients with TIA without first identifying a high-risk category medical therapy medical therapy. Moreover, the link between increased risk with PTAS and conditions like hypertension, diabetes, hyperlipidaemia and latest ischaemic event to randomisation <30 days calls for further research and has significant implications for future study designs.
The main outcomes were not significantly different between PTAS and medical therapy for symptomatic ICA, MCA and VA stenosis. This underscores that medical therapy should continue to be the preferred strategy for these types of stenosis. However, PTAS exhibited a numerically lower rate of the primary outcomes in MCA and VA stenosis patients, highlighting the potential need for a more comprehensive screening system to identify subgroups that might benefit from PTAS. In both the CASSISS and SAMMPRIS trials, participants had to have a major intracranial artery with 70%–99% atherosclerotic stenosis, as shown by catheter angiography. To maximise the benefits of PTAS, relying solely on stenosis degree for patient evaluation may not be adequate; a more sophisticated assessment system is warranted. Potential techniques could involve high-resolution magnetic resonance vessel wall imaging to evaluate plaque stability, the risk associated with perforating arteries and haemodynamic deficits.18 These assessments are crucial for identifying patients who require PTAS. The SAMMPRIS trial demonstrated that 40% of patients exhibiting greater than 70% luminal stenosis presented with a fractional flow reserve (FFR)≤0.8, underscoring the significance of integrating haemodynamic and morphological parameters in assessing the necessity for endovascular intervention. FFR measurements provide a functional assessment of ICAS severity and assist in clinical decision-making.19 Haemodynamic measurements are instrumental in identifying ICAS patients who require endovascular interventions, thus facilitating the refinement of clinical treatment protocols. Furthermore, a comprehensive analysis indicated lower occurrences of short-term stroke or mortality (peri-procedural or average follow-up ≤3 months) associated with balloon angioplasty in comparison to stenting.20 Consequently, balloon angioplasty may serve as an effective standalone option for treating symptomatic ICAS, supported by an ongoing prospective, multicentre, randomised, controlled trial aimed at evaluating its therapeutic outcomes.21 Additionally, the adoption of submaximal balloon angioplasty, known for diminishing the risks associated with thromboembolism, vessel rupture and reperfusion bleeding, is favoured by numerous clinicians as the method of choice when performing angioplasty exclusively.22–24
In the SAMMPRIS subgroup analysis, the incidence of the primary outcome for VA stenosis was notably higher in the PTAS group, while it was relatively comparable between the two groups in this study. The inclusion of a larger sample size in the present study likely facilitated a more accurate estimation of differences between the groups, even if these differences were not statistically significant. This finding was corroborated by an analysis of individual patient data regarding stenting for symptomatic VA stenosis as well as a post hoc examination of CASSISS.25 26
This study confirmed that different qualifying arteries do not impact the efficacy of medical therapy for symptomatic ICAS patients. However, when treated with PTAS, the incidence of ICA (26.67%) and BA (17.17%) was significantly higher than that of MCA (8.28%) and VA (4.76%). Regarding ICA stenosis, increased ICAS distortion may enhance the likelihood of vessel wall damage, leading to inadequate stent deployment and subsequent haemodynamic changes. These factors may explain the high incidence. In cases of BA stenosis, it is probable that stenting disrupts blood flow in the perforating vessels.15 Moreover, our research revealed that the posterior circulation exhibited a greater frequency of the primary outcome compared with the anterior circulation in patients with symptomatic ICAS who underwent PTAS, aligning with the findings of Gröschel et al.27 However, our study lacked data on the specific mechanisms driving the occurrence of adverse events in individual patients. The notable differences in the incidence of different qualifying arteries warrant further investigation. These results suggest that exploring clinical outcomes based on different qualifying arteries is crucial for tailoring treatment.
The robustness of this analysis is attributed to the incorporation of individual patient data, facilitating a thorough evaluation of advantages for both the general population and specific subgroups. SAMMPRIS included very few patients of Asian ethnicity, whereas CASSISS included a large number of Asian patients, enhancing the breadth of interpreting and generalising the results. However, several limitations need to be addressed. First, although this study included data from both trials, the power of the analysis was relatively weak, partly because the SAMMPRIS trial was terminated early by the funders. Second, the study did not distinguish between patients with a first-time stroke or TIA and those with a recurrent, medically resistant stroke or TIA, which are often indications for stenting. Thirdly, given the advancements in PTAS surgery, including equipment, techniques, patient selection, and intervention timing, the applicability of these older data to current medical therapy practice requires further validation. Fourthly, in the BA stenosis subgroup analysis, the wide 95% CI indicate that the results were underpowered and require further confirmation. Lastly, slight differences in the inclusion and exclusion criteria between the SAMMPRIS and CASSISS studies could have introduced bias, impacting the reliability of the conclusions.
Conclusions
This retrospective analysis of data from the SAMMPRIS and CASSISS trials offers the most detailed evaluation to date of PTAS versus medical therapy alone in symptomatic ICAS affecting diverse arterial segments medical therapy alone. PTAS for BA stenosis demonstrates a notably increased risk for both short-term and long-term stroke. The potential for PTAS to provide significant advantages remains limited until future technological developments can considerably mitigate the stroke risks inherent to its application. This research does not confirm the benefits of PTAS for treating stenosis in the ICA, MCA and VA. These insights will guide the design of forthcoming clinical trials and therapeutic strategies for symptomatic ICAS.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This is a pooled analysis of CASSISS and SAMMPRIS Trials. The protocols for individual trials were approved at sites’ local institutional review boards, and all studies were registered at clinicaltrials.gov. All participants and/or their legally authorised representatives provided informed consent before enrolment in the individual studies. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We thank the patients and their families for participating in this trial.
Footnotes
TL, JL and XB contributed equally.
Contributors LJ and TW led study design. TLi, XB and JL drafted the manuscript. EA and CD contributed to study design and all authors critically revised the manuscript. TLi did the statistical analyses. PG, DL, RX, WX, GLu, HG, XZ, TLu, JW, RY, ZX, GLiu and YD accessed and verified the data underlying this article. LJ and TW accept full responsibility for the work and the conduct of the study as the guarantor, had access to the data, and controlled the decision to publish.
Funding This work was supported by Beijing Hospitals Authority’s Ascent Plan (DFL20220702) and National Natural Science Foundation of China (82101398).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer-reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.