Abstract
Background The benefit of intravenous alteplase in acute ischaemic stroke (AIS) is time-dependent. Tenecteplase is non-inferior to alteplase among patients with AIS. We aimed to delineate the association of the stroke onset to treatment time (OTT) with tenecteplase compared with alteplase on therapeutic benefit and clinical risks.
Methods This is a post hoc analysis of the Tenecteplase Reperfusion therapy in Acute ischaemic Cerebrovascular Events-2 an open-label, randomised, controlled, non-inferior trial. A total of 1430 AIS within 4.5 hours onset at 53 sites in China from 12 June 2021 to 29 May 2022 were randomly assigned (1:1) to receive either tenecteplase 0.25 mg/kg or alteplase 0.9 mg/kg. The primary efficacy outcome was the proportion of participants with a modified Rankin Scale score of 0–1 at 90 days. A post hoc subgroup analysis was conducted with the OTT divided into three intervals (0–90 min, 91–180 min and 181–270 min). The primary safety outcome was symptomatic intracranial haemorrhage within 36 hours post-thrombolytic treatment.
Results Treatment was initiated within 270 min of stroke onset in 1412 patients who were randomly allocated to either tenecteplase (n=707) or alteplase (n=705). The OR of primary efficacy outcome was similar as OTT increased (p=0.84). Adjusted odds of an excellent functional outcome were 0.99 (95% CI 0.37 to 2.67) for 0–90 min, 1.23 (95% CI 0.88 to 1.71) for 91–180 min and 1.21 (95% CI 0.88 to 1.65) for 181–270 min. All were in favour of the tenecteplase group. Meta-analysis of 2949 patients yielded a pooled risk difference of 5.54 (95% CI −0.18 to 11.26; p=0.82) in favour of tenecteplase for more than 180 min and 1.77 (95% CI −2.66 to 6.20; p=0.58) for 0–180 min.
Conclusions In AIS patients who were treated with either tenecteplase or alteplase within 4.5 hours onset, there was no difference observed in the efficacy and safety between the two groups at the three different OTT time intervals.
WHAT IS ALREADY KNOWN ON THIS TOPIC
The Tenecteplase Reperfusion therapy in Acute ischaemic Cerebrovascular Events-2 (TRACE-2) trial (NCT04797013) of 1430 subjects and the Alteplase Compared with Tenecteplase in Patients With Acute Ischaemic Stroke (AcT) trial (NCT 03889249) of 1600 subjects showed noninferiority of tenecteplase 0·25 mg/kg to alteplase among patients with AIS. The benefit of intravenous alteplase in acute ischaemic stroke (AIS) is time-dependent and decrease as the time from stroke onset to treatment (OTT) increases. There is a paucity of data on the relationship of OTT with intravenous tenecteplase in comparison with alteplase on excellent functional outcome at 90 days.
WHAT THIS STUDY ADDS
This subgroup analysis of TRACE-2 provided additional evidence that there is a correlation between the treatment time and benefits of tenecteplase compared with alteplase at three time intervals (0–90 min, 91–180 min and 181–270 min) within 4.5 hours. Although there was a numerically higher proportion of patients with an excellent functional outcome in the tenecteplase group than in alteplase group if treated beyond 90 min, no significant differences were observed between the two treatment groups.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study found that there were no significant differences in the efficacy or safety between tenecteplase and alteplase for patients with AIS regardless of the OTT. Definitive evidence of the practical advantages of tenecteplase for intravenous thrombolysis is still lacking, but evidence of non-inferiority is sufficient to support the routine use of tenecteplase in this population in lieu of alteplase for reasons of cost or convenience.
Introduction
Intravenous alteplase is beneficial for patients with acute ischaemic stroke (AIS) within 4.5 hours.1–3 Tenecteplase is a potential alternative thrombolytic agent for the treatment of AIS. The are four major potential benefits in clinical practice to using tenecteplase over alteplase: (1) the potential for shorter door-to-needle times due to a single bolus injection, (2) avoiding the risk of delay between alteplase bolus and infusion which can reduce peak drug concentrations and efficacy, (3) lower cost in some parts of the world and (4) the opportunity for shorter door-in door-out times when patients must be transferred to another centre for thrombectomy.4 Two phase 3, randomised controlled trials, Tenecteplase in Patients With Acute Ischaemic Stroke (AcT) trial (NCT 03889249)5 and the Tenecteplase Reperfusion therapy in Acute ischaemic Cerebrovascular Events-2 (TRACE-2) trial (NCT 04797013),6 have demonstrated the non-inferiority of tenecteplase 0.25 mg/kg to standard-of-care alteplase among patients with AIS within 4.5 hours of symptom onset.
The benefits are time-dependent and decrease as the time from stroke onset to treatment (OTT) increases.7–10 It remains unclear if there is an effect of time to treatment with tenecteplase compared with alteplase. Since the convenient clinical practice of tenecteplase over alteplase, there is a hypothesis that in comparison with alteplase, tenecteplase may get more excellent functional outcome at 90 days in patients with moderate to severe stroke who arrive at the emergency at later time points, despite an augmented risk of haemorrhage.
The TRACE-2 trial demonstrated that tenecteplase exhibited non-inferiority to alteplase for patients with AIS when treated within 4.5 hours of stroke onset who meet the conditions for intravenous thrombolysis (IVT) but not for endovascular thrombectomy (EVT). In this post hoc analysis of TRACE-2, we aimed to delineate the therapeutic benefit and clinical risks of tenecteplase compared with alteplase within three different OTT intervels (0–90 min, 91–180 min and 181–270 min).
Methods
Overview of the TRACE-2 trial
The TRACE-2 trial was a multicentre, prospective, randomised, open-label, blinded endpoint, controlled phase 3 trial done at 53 study sites in China. The objective of the trial was to demonstrate that tenecteplase is not inferior to alteplase for patients with AIS within 4.5 hours of symptom onset (online supplemental study protocal).11 Statistical analysis plan and the primary results of the trial have been published previously.
Supplementary data
In brief, eligible patients were those with an AIS within 4.5 hours who were eligible for IVT but ineligible for EVT.
Each patient was given either intravenous tenecteplase (0.25 mg/kg, max dose 25 mg, single bolus) or intravenous alteplase (0.9 mg/kg, max dose 90 mg, 10% of dose as initial bolus, followed by 90% in an hour infusion). Treatment was open labelled. Other clinical management was performed by the local neurologists according to the guidelines.1 Patients were followed up for 90 days after randomisation. Clinical assessments during the follow-up were performed at local site by blinded evaluators who had received comprehensive training and certification.
The primary outcome of the TRACE-2 trial was the proportion of the patients with an excellent functional outcome defined as a modified Rankin Scale (mRS) of 0–1 at 90 days. The primary safety outcome was the incidence of symptomatic intracranial haemorrhage (sICH) within 36 hours as defined in the European Cooperative Acute Stroke Study III.12 The primary efficacy and safety outcomes of this post hoc subgroup analysis was the same as those of the TRACE-2 trial analysis. Consistent with the primary article, improvement of neurological function in National Institutes of Health Stroke Scale (NIHSS) is defined as reducing by at least 4 points or a score no more than 1 and symptomatic parenchymal haematoma 2 (PH2) within 36 hours as defined by Safe Implementation of Thrombolysis in Stroke-Monitoring Study. An independent, blinded clinical event committee, adjudicated the clinical outcome events using rigorous prespecified outcome definitions.
Subgroup analysis
In this study, we conducted a post hoc subgroup analysis of TRACE-2 to assess the primary and secondary outcomes, and safety by three OTT intervals. The three OTT intervals were defined as 0–90 min, 91–180 min and 181–270 min according to the definition previously used in many clinical trials.7–10
Search strategy and selection criteria of meta-analysis
We also performed a systematic review and meta-analysis of Act trial and TRACE-2 trial. The two trials were randomised clinical trials in which AIS patients within 4.5 hours onset were assigned to either tenecteplase or alteplase.
Statistical analysis
Continuous variables were reported as median with IQR, and categorical variables as frequencies and percentages. Baseline characteristics between tenecteplase and alteplase in different OTT subgroups were compared by Kruskal-Wallis test for continuous variables and χ2 for categorical variables. Differences in the primary outcomes between tenecteplase and alteplase among three intervals of OTT were assessed using binary logistic regression, and OR, as well as its 95% CI, was reported. Similar approaches were used for the comparison of the secondary outcomes and of the safety outcomes, and ordinal logistic regression for the ordinal mRS score at 3 months was performed with common OR and 95% CI reported. An unadjusted model and a model adjusted for age, sex and weight were estimated for the efficacy outcomes. The interaction between treatment and OTT on efficacy outcomes was performed with tests for trend by entering the median value of each category of OTT as a continuous variable and its interaction with treatment in the models. To achieve maximum study power to investigate whether the effect of tenecteplase compared with alteplase on the primary outcome changes with OTT, an interaction between OTT by treatment interaction treating time as a continuum was tested. Subgroup analyses were then explored to examine whether the differences in the primary outcome between tenecteplase and alteplase in different OTT subgroups differed by stroke severity.
For the meta-analysis, we used data of risk difference and 95% CI for the excellent functional outcome. A random-effect model (DerSimonian-Laird) was performed to pool the data for the outcome.
The meta-analysis was performed with Stata software V.13.0 (StataCorp), and the other statistical analyses were performed with SAS statistical software, V.9.4 (SAS Institute). All tests were two sided, and a p<0.05 was considered statistically significant.
Results
Between June 2021 and June 2022, 1430 AIS patients eligible for IVT but ineligible for EVT were enrolled in TRACE-2 trial and assigned to receive either tenecteplase (n=716) or alteplase (n=714) (table 1). The modified intention-to-treat population included 710 patients assigned to the tenecteplase group and 707 patients in the alteplase group. Of those, 1412 (99.6%) with OTT time ranging from 10 to 270 min were analysed in this subgroup analysis (online supplemental figure 1). Baseline characteristics of patients based on the OTT intervals are shown in online supplemental table 1. There were 84 (5.9%) patients with OTT time of 0–90 min (median 75 min; IQR 65–84), 622 (43.9%) patients with OTT time of 91–180 min (median 140 min; IQR 117–161) and 706 (49.8%) patients with OTT time of 181–270 min (median 226 min; IQR 204–248). The treatment groups had a good balance in terms of gender, baseline stroke severity, medical history and door-to-needle time. In the OTT time of 91–180 min interval, patients in the tenecteplase group were older (66 years vs 64.3 years, p=0.03) and weighed less (66 kg vs 68 kg, p=0.04) than patients in the alteplase group. In the OTT time of 181–270 min interval, the onset-to-needle time of tenecteplase group (221.5 min, IQR, 200–245) was shorter than alteplase group (230 min, IQR, 209–250; p=0.005).
Supplementary data
Baseline characteristics of patients grouped by onset to treatment times
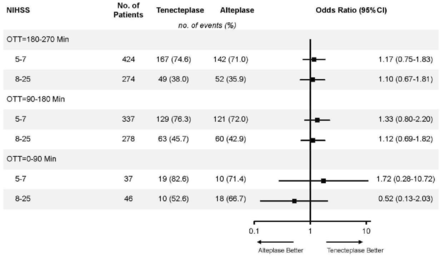
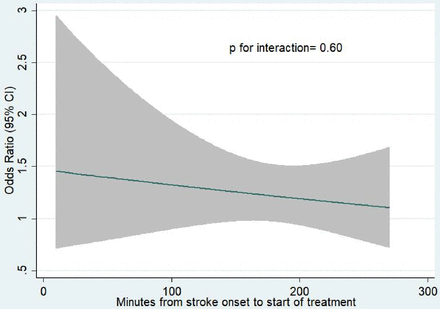
Overall, there was no statistical difference in the relationship of OTT with tenecteplase versus alteplase for the primary efficacy outcome (mRS of 0–1) at 90 days (p=0.84) (table 2). Primary outcome was achieved in 29 (69.1%) of 42 patients in the tenecteplase group and 28 (68.3%) of 41 patients in the alteplase group (adjusted OR 0.99, 95% CI 0.37 to 2.67; p=0.98) with OTT time of 0–90 min, 192 (62.5%) of 307 patients in the tenecteplase group and 181 (58.8%) of 308 patients in the alteplase group (adjusted OR 1.23, 95% CI 0.88 to 1.71; p=0.22) with OTT time of 91–180 min and 216 (61.2%) of 353 patients in the tenecteplase group and 194 (56.2%) of 345 patients in the alteplase group (adjusted OR 1.21, 95% CI 0.88 to 1.65; p=0.23) with OTT time of 181–270 min. Similar results were observed in secondary outcomes including mRS of 0–2 at 90 days (p=0.94), ordinal mRS score at 90 days (p=0.85), improvement of neurological function in NIHSS at 24 hours (p=0.20), and at 7 days or discharge whichever occurs first (p=0.06)) and the Barthel index ≥95 points at 90 days (p=0.66). No significant differences in the relationship of OTT with tenecteplase versus alteplase among patients with a different stroke severity were observed (figure 1). The results of the interaction between OTT by treatment interaction treating time as a continuum are shown in figure 2.
Efficacy outcomes at 3 months grouped by onset to treatment times
Forest plot of randomised comparisons of intravenous tenecteplase or alteplase for patients with AIS within 4.5 hours of stroke onset. AIS, acute ischaemic stroke; NIHSS, National Institutes of Health Stroke Scale; OTT, onset to treatment time.
Model estimating OR for excellent outcome at 3 months in tenecteplase-treated patients compared with alteplase-treated patients by OTT. OTT, onset to treatment time.
There were no sICH events in the OTT time of 0–90 min. Symptomatic ICH within 36 hours occurred in 7 (2.3%) of 309 patients in the tenecteplase group and 5 (1.6%) of 313 patients in the alteplase group (OR 1.43, 95% CI 0.45 to 4.55; p=0.55) in the OTT time of 91–180 min, and in 8 (2.3%) of 356 patients in the tenecteplase group and 8 (2.3%) of 350 patients in the alteplase group (OR 0.98, 95% CI 0.37 to 2.65; p=0.97) in the OTT time of 181–270 min (table 3). Among all sICH, PH2 within 36 hours occurred in 5 (1.6%) patients in the tenecteplase group and no patients in the alteplase group in the OTT time of 91–180 min and 5 (1.4%) of patients in the tenecteplase group and 3 (0.9%) in the alteplase group with OTT time of 181–270 min. Death within 90 days occurred in 3 patients (7.0%) in the tenecteplase group and 1 (2.4%) patient in the alteplase group in the OTT time of 0–90 min, 16 (5.2%) patients in the tenecteplase group and 12 (3.8%) patients in the alteplase group in the OTT time of 91–180 min and 26 (7.3%) patients in the tenecteplase group and 22 (6.3%) patients in the alteplase group in the OTT time of 181–270 min. The incidence of adverse events and serious adverse events was similar between the two groups in different OTT intervals.
Safety outcomes at 3 months grouped by onset to treatment times
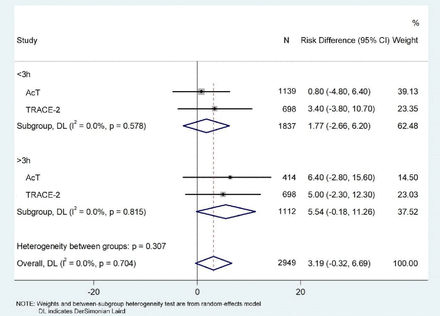
There were only two studies, TRACT-2 and AcT, and were eligible for the meta-analysis. In the meta-analysis, we pooled 2949 patients with AIS treated within 4.5 hours of onset and randomly assigned to receive tenecteplase 0.25 mg/kg or alteplase 0.9 mg/kg, and yielded a pooled risk difference of 3.19 (95% CI −0.32 to 6.69; p=0.704) for excellent functional outcome in favour of tenecteplase, without evidence of heterogeneity (I2=0%) (figure 3). Moreover, a pooled risk difference of 5.54 (95% CI −0.18 to 11.26; p=0.82) in favour of tenecteplase for more than 180 min and 1.77 (95% CI −2.66 to 6.20; p=0.58) for 0–180 min.
Pooled risk difference for modified Rankin Scale Scores 0–1 at 90 days by OTT and stroke severity. OTT, onset to treatment time; TRACE-2, Tenecteplase Reperfusion therapy in Acute ischaemic Cerebrovascular Events-2.
Discussion
In this subgroup analysis of the TRACE-2 trial, we found that there was no difference in the efficacy and safety between tenecteplase 0.25 mg/kg and alteplase 0.9 mg/kg for excellent functional outcome at 90 days when the OTT time was analysed using three different time intervals within 4.5 hours onset. Although there was a numerically higher proportion of patients with an excellent functional outcome in the tenecteplase group than in alteplase group if treated beyond 90 min, no significant differences were observed between the two treatment groups.
Early thrombolytic treatment brings more benefits. A pooled analysis of eight randomised placebo-controlled trials showed that earlier treatment with intravenous alteplase was associated with greater benefit at 90 days.8 The time window of benefit from intravenous alteplase, selected patients with ischaemic stroke by clinical symptoms and CT, was up to 4.5 hours. The Get With The Guidelines-Stroke Programme, which is a hospital-based clinical quality registry cohort study representing US clinical practice, has published similar findings.10 Tenecteplase, administered as a single intravenous bolus, may confer additional clinical benefits because of its practical advantages.13–15 The convenient administration of tenecteplase as a bolus may achieve a more rapid thrombolytic effect of the occluded artery. Basic research indicated that tenecteplase produced faster and more complete recanalisation of occluded arteries in a rabbit experimental model compared with alteplase.16 Our findings provided clinical data on the correlation between OTT and the benefits of tenecteplase and alteplase. Consistent with previous trials, we observed that the proportion of excellent functional outcome decreased with time in both the alteplase and tenecteplase groups. We did not observe a greater benefit in clinical outcomes with tenecteplase in the late time window despite the ease of administration. The adjusted OR of excellent functional outcome for patients treated with tenecteplase compared with alteplase was 1.23 for those treated within 91–180 min and 1.21 for those treated within 181–270 min.
Since TRACE-2 trial was a non-inferiority study, the power of these subgroup analyses was limited. To address this limitation, we performed a meta-analysis with data from the AcT trial, a randomised clinical phase 3 trial of tenecteplase versus alteplase. No significant differences were seen in 90-day excellent functional outcome despite the increased sample size, meta-analysis estimates suggest that there was an increase in excellent outcomes with tenecteplase versus alteplase with increasing OTT. Definitive evidence of the practical advantages of tenecteplase for IVT is still lacking, but evidence of non-inferiority is sufficient to support the routine use of tenecteplase in this population in lieu of alteplase for reasons of cost or convenience.
Previous IVT studies in the last two decades showed that patients with more severe strokes arrived earlier in the emergency department.7 10 In the TRACE-2 trial, stroke severity was similar in the three OTT intervals. Up to now, there is no evidence suggesting that patients arrived at the emergency at later time window had more excellent functional outcome at 90 days with tenecteplase compared with alteplase. In our study, the results showed that alteplase had higher rate of improvement on NIHSS of ≥4 points or a score ≤1 at 24 hours and at 7 days or discharge than tenecteplase in patients who arrived at 0–90 min time window. However, no significant difference of improvement on NIHSS score was found between the tenecteplase and alteplase at later time window, which is theoretically more likely to show the difference. Therefore, we cannot exclude the possibility that the higher rate of improvement showed in alteplase might be a chance finding due to the small sample (less than 85 patients). Whether alteplase will have more improvement on NIHSS of ≥4 points or a score ≤1 at 24 hours, 7 days or discharge than tenecteplase in patients treated within 0–90 min needs to be further validated in larger populations.
In our study, we did not observe an obvious faster door-to-needle time of tenecteplase compared with alteplase. However, tenecteplase does save about an hour of administration time that allows the full dose to be administered at once and might have a faster onset of therapeutic concentrations.17 A meta-analysis of five randomised clinical trials of patients with large vessel occlusion ischaemic stroke showed that for every 15 min faster door-to-reperfusion time, an estimated 25 patients would achieve functional independence (defined as mRS 0–2).18 Our study did not show whether the 1-hour reduction in tenecteplase administration time benefits patients with large-vessel occlusion, especially those requiring IVT treatment. Therefore, the practical advantages in administration of tenecteplase deserve to be assessed in AIS patients eligible for IVT.
Several limitations should be considered in this study. First, we might not have sufficient samples to determine the shape of the relation between time to treatment and treatment effect. Second, The inclusion of patients who met the exclusion criteria for eligibility for EVT resulted in a selection bias, leading to a predominantly mild stroke severity among the included individuals. Third, incomplete baseline multimodal CT or multimodal MRI limited our ability to explore informative secondary endpoints.
In conclusion, this subgroup analysis provided additional evidence regarding the association between treatment time and the benefits of tenecteplase compared with alteplase at three time intervals within 4.5 hours. The results showed no significant difference in the efficacy and safety between tenecteplase as compared with alteplase for any OTT. The ‘golden hour’ of efficacy was present in both tenecteplase and alteplase.
Data availability statement
Data sharing not applicable as no data sets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and the protocol of this study was reviewed and approved by Institutional Review Board of Tiantan Hospital and each centre. Participants gave informed consent to participate in the study before taking part.
Footnotes
X @braindoc_mgh
Contributors YoW had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis and is responsible for the overall content as the guarantor . Concept and design: SL, HL and YiW. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: SL and RW. Critical revision of the manuscript for important intellectual content: LHS, MF, BCVC, MP and DW. Statistical analysis: AJ. Obtained funding: YW.
Funding This study was supported by National Natural Science Foundation of China (81870905, U20A20358, 82111530203). Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5-029). The National Science and Technology Major Project (2017ZX09304018).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.