Abstract
Objectives Evidence of the optimal antiplatelet therapy for elderly patients who had a stroke is limited, especially those elder than 80 years. This study aimed to explore the efficacy and safety of dual antiplatelet therapy (DAPT) in old-old patients compared with younger patients in the ticagrelor or Clopidogrel with aspirin in High-risk patients with Acute Non-disabling Cerebrovascular Events-II (CHANCE-2) trial.
Methods CHANCE-2 was a randomised, double-blind, placebo-controlled trial in China involving patients with high-risk transient ischaemic attack or minor stroke with CYP2C19 loss-of-function alleles. In our substudy, all enrolled patients were stratified by age: old-old (≥80 years), young-old (65–80 years) and younger (<65 years). The primary outcomes were stroke recurrence and moderate to severe bleeding within 90 days, respectively.
Results Of all the 6412 patients, 406 (6.3%) were old-old, 2755 (43.0%) were young-old and 3251 (50.7%) were younger. Old-old patients were associated with higher composite vascular events (HR 1.41, 95% CI 1.00 to 1.98, p=0.048), disabling stroke (OR 2.43, 95% CI 1.52 to 3.88, p=0.0002), severe or moderate bleeding (HR 8.40, 95% CI 1.95 to 36.21, p=0.004) and mortality (HR 7.56, 95% CI 2.23 to 25.70, p=0.001) within 90 days. Ticagrelor-aspirin group was associated with lower risks of stroke recurrence within 90 days in younger patients (HR 0.68, 95% CI 0.51 to 0.91, p=0.008), which was no differences in old-old patients.
Conclusion Elderly patients aged over 80 in CHANCE-2 trial had higher risks of composite vascular events, disabling stroke, severe or moderate bleeding and mortality within 90 days. Genotype-guided DAPT might not be as effective in old-old patients as in younger ones.
Trial registration number NCT04078737.
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is limited scientific evidence regarding the optimal antiplatelet therapy regimen for elderly patients aged ≥80, who are largely excluded from most trials and registry studies owing to age constraints in the study design or multiple comorbidities/complications in these patients.
WHAT THIS STUDY ADDS
Elderly patients aged ≥80 in the ticagrelor or Clopidogrel with aspirin in High-risk patients with Acute Nondisabling Cerebrovascular Events-II trial had higher risks of composite vascular events, disabling stroke, severe or moderate bleeding and mortality within 90 days.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Genotype-guided dual antiplatelet therapy might not be as effective in old-old patients as in younger ones.
Introduction
Epidemiological changes in the field of stroke pose significant challenges to international health services. Continued population growth and ageing were associated with the increasing number of ischaemic stroke cases.1–5 The incidence of stroke might increase by 30% within the next decade, particularly in elderly patients, who are expected to survive longer than previously expected.6 Age is an independent predictor of poor outcomes in ischaemic stroke.7 8 Elderly patients who had a stroke might have more severe symptoms, more comorbidities (eg, pulmonary infection and cognitive impairment), higher mortality and poorer prognosis compared with younger patients.9 These changes will generate huge clinical and financial influence in medical practice.
However, elderly patients were not as active as young patients in receiving secondary prevention treatment, which might be due to concerns about adverse drug reactions (such as gastrointestinal adverse events) and bleeding risk.10 Clinicians were also more conservative in prescribing antiplatelet therapy to elderly patients for the same reason.11–14 Additionally, there is limited scientific evidence regarding the optimal antiplatelet therapy regimen for elderly patients aged over 80 years, who are largely excluded from most randomised clinical trials and registry studies owing to age constraints in the study design or multiple comorbidities/complications in these patients.15–17 Furthermore, existing guidelines for ischaemic stroke are not generally applicable to these elderly patients.18 19 Therefore, it is of great clinical and scientific importance to explore the efficacy and safety of antiplatelet therapy in elderly patients in high-quality large-scale randomised controlled clinical studies.
The CHANCE-2 (ticagrelor or Clopidogrel with aspirin in High-risk patients with Acute Non-disabling Cerebrovascular Events II) trial was a randomised trial that evaluated the efficacy and safety of dual antiplatelet therapy (DAPT) with ticagrelor and aspirin (ticagrelor-aspirin) versus clopidogrel and aspirin (clopidogrel-aspirin) in patients with minor ischaemic stroke or high-risk transient ischaemic attack (TIA) who were CYP2C19 loss-of-function (LOF) alleles carriers.20 CHANCE-2 demonstrated that ticagrelor-aspirin was superior to clopidogrel-aspirin for reducing stroke recurrence in Chinese patients with minor ischaemic stroke or TIA at 90 days.
In this subgroup analysis of the CHANCE-2 trial, we aimed to investigate the efficacy and safety of DAPT in old-old patients (≥80 years) compared with young-old patients (65–80 years) and younger patients (<65 years) with minor stroke or TIA.
Methods
Study population
CHANCE-2 was an investigator-initiated, multicentre, randomised, double-blind, placebo-controlled trial. The detailed study design and data have been reported previously.20 Baseline characteristics included demographic characteristics, smoking status, vascular risk factors, baseline NIHSS and ABCD2 scores, etc. From 2019 to 2021, the entire study recruited 6412 patients and assigned to intervention group (ticagrelor-aspirin) and control group (clopidogrel-aspirin). Patients in the intervention group began with placebo clopidogrel and loading dose of ticagrelor on the first day. On the days 2–90, the patients were administered ticagrelor 90 mg two times per day. Patients in the control group began with placebo ticagrelor and loading dose of clopidogrel on the first day. On the days 2–90, the patients were administered clopidogrel 75 mg daily. Both groups were given 75–300 mg of aspirin the first day, and followed by 75 mg per day over 21 days.21
The NIHSS and ABCD2 on admission were evaluated by well-trained neuroscientists. Neuroscientists who collected data via face-to-face interview did not know the distribution of group.
In this subgroup analysis, we evaluated the efficacy and safety of DAPT in patients stratified by age: old-old patients (≥80 years), young-old patients (65–80 years) and younger patients who had a stroke (<65 years).
Outcome assessment
The main outcome was a new ischaemic or haemorrhagic stroke within 90 days for efficacy and severe or moderate bleeding for safety within 90 days. The secondary outcomes included new stroke within 30 days, composite vascular events (stroke, TIA, myocardial infarction and vascular death), ischaemic stroke, disabling stroke (with a subsequent mRS score of 2 or higher; range 0–6 with higher scores reflecting greater handicap), any bleeding, intracranial haemorrhage and mortality through 90 days of follow-up.
Statistical analysis
Continuous variables were presented as medians with IQRs and categorical variables were presented as frequencies and percentages. The baseline characteristics between different age subgroups were compared by Kruskal-Wallis test for continuous variables and χ2 for categorical variables. The cumulative risks of the primary outcome of any ischaemic or haemorrhagic event during the 90-day follow-up were estimated from Kaplan-Meier plots.
Differences in the efficacy and safety outcomes during the 90-day follow-up were assessed using a Cox proportional hazards regression model, and HRs with 95% CIs were reported. The interaction of treatment assignment was evaluated with the addition of age category in a Cox model. Confounding factors were selected if the univariate analysis revealed statistically significant differences at baseline, such as age, sex, medical history and previous treatments. All statistical analyses were performed with SAS statistical software, V.9.4 (SAS Institute). All tests were two sided and a p<0.05 was considered statistically significant.
Results
Baseline characteristics
There were 6412 patients with minor stroke or TIA were included in our study, among which 406 (6.3%) patients were old-old (≥80 years), 2755 (43.0%) patients were young-old (65–80 years) and 3251 (50.7%) patients were younger (<65 years).
The baseline characteristics stratified by age are shown in tables 1 and 2. Old-old patients were more female, had more history of myocardial infarction, had more previous antiplatelet therapy and lipid-lowering therapy, had more symptomatic intracranial and extracranial artery stenosis, were less likely to have diabetes mellitus and dyslipidaemia, and had less current smoking and drinking (table 1). After combining with different dual antiplatelet treatment group, old-old patients distributed to ticagrelor-aspirin group had more history of diabetes, more current drinking and less history of TIA (table 2).
Baseline characteristics of the patients stratified by age
Baseline characteristics of the patients stratified by age and ticagrelor-aspirin versus clopidogrel-aspirin
Outcomes
Regarding the efficacy outcomes, old-old patients were associated with an increasing rate of stroke recurrence within 90 days (9.6% vs 6.8% vs 6.4%), stroke within 30 days (8.1% vs 5.7% vs 5.3%), ischaemic stroke (9.4% vs 6.8% vs 6.2%), composite vascular events (11.6% vs 8.3% vs 7.6%) and disabling stroke (7.1% vs 2.8% vs 2.5%) within 90 days. After adjusting for multiple factors, old-old patients remained associated with a higher risk of composite vascular events (HR 1.41, 95% CI 1.00 to 1.98, p=0.048) and disabling stroke (OR 2.43, 95% CI 1.52 to 3.88, p=0.0002) within 90 days (table 3).
Efficacy and safety outcomes stratified by age
Regarding the safety outcomes, old-old patients had higher rates of severe or moderate bleeding (1.0% vs 0.3% vs 0.2%), any bleeding (4.4% vs 3.4% vs 4.2%), intracranial haemorrhage (0.5% vs 0.1% vs 0.2%) and mortality (1.5% vs 0.4% vs 0.3%) within 90 days. After adjusting for multiple factors, there were still statistically significant differences of severe or moderate bleeding (HR 8.40, 95% CI 1.95 to 36.21, p=0.004) and mortality (HR 7.56, 95% CI 2.23 to 25.70, p=0.001) at 90 days (table 3).
As shown in table 4, in consideration of different DAPT groups (ticagrelor-aspirin group or clopidogrel-aspirin group), old-old patients did not exhibit significant differences for the efficacy and safety outcomes after adjustment, including new stroke within 90 days (HR 1.00, 95% CI 0.49 to 2.06, p=0.99), new stroke within 30 days (HR 1.11, 95% CI 0.50 to 2.48, p=0.80), ischaemic stroke (HR 0.94, 95% CI 0.45 to 1.95, p=0.86), composite vascular events (HR 0.93, 95% CI 0.47 to 1.83, p=0.83), disabling stroke (OR 1.00, 95% CI 0.44 to 2.27, p=0.99), severe or moderate bleeding (HR 0.80, 95% CI 0.06 to 11.35, p=0.87), any bleeding (HR 1.78, 95% CI 0.48 to 6.66, p=0.39) and mortality (HR 0.08, 95% CI 0.01 to 1.37, p=0.08) within 90 days. In contrast to the findings for old-old patients, in younger patients, ticagrelor-aspirin was associated with lower risks of stroke within 90 days (HR 0.68, 95% CI 0.51 to 0.91, p=0.008), stroke within 30 days (HR 0.67, 95% CI 0.49 to 0.92, p=0.01), ischaemic stroke within 90 days (HR 0.71, 95% CI 0.53 to 0.94, p=0.02) and composite vascular events within 90 days (HR 0.71, 95% CI 0.55 to 0.92, p=0.01) compared with clopidogrel-aspirin. As age increased as a continuous variable (online supplemental appendix 1), we found that the combination of clopidogrel and aspirin was not inferior to the combination of ticagrelor and aspirin with regard to the risk of stroke in elderly patients, as CIs were rather broad among the elderly.
Supplementary data
Association of ticagrelor-aspirin versus clopidogrel-aspirin with efficacy and safety outcomes stratified by age
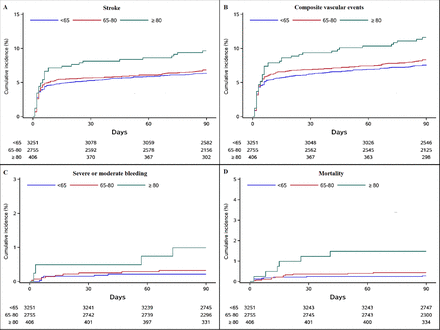
The cumulative risks of the efficacy and safety outcomes among patients with different status of age were shown in figure 1. The cumulative risks of the efficacy and safety outcomes among patients with different status of age and treatment assignment were shown in online supplemental appendix 2.
Kaplan-Meier curves of the efficacy and safety outcomes in patients with different ages.
Discussion
In this subgroup analysis of the CHANCE-2 trial, we found that old-old patients with minor stroke or TIA receiving DAPT had higher risks of composite vascular events, disabling stroke, severe or moderate bleeding and mortality within 90 days compared with younger patients. For old-old patients, there were no differences in the efficacy and safety outcomes between different DAPT regimens (ticagrelor-aspirin vs clopidogrel-aspirin).
Antiplatelet therapy is the golden standard for acute and secondary prevention of ischaemic stroke.22 23 Multiple randomised clinical trials have shown that DAPT is more effective than single antiplatelet therapy in reducing stroke recurrence in patients with ischaemic stroke or TIA.24 25 Recently, the CHANCE-2 trial found that ticagrelor-aspirin was superior to clopidogrel-aspirin for stroke prevention in patients with minor stroke or TIA.20 However, there is a lack of clinical and scientific evidence about the acute and secondary antiplatelet therapies for elderly patients aged ≥80 years, given that these patients are underrepresented or excluded in most stroke clinical studies and trials.16 17 Precise assessment of the efficacy and safety of antiplatelet therapy for elderly patients was more important than ever to improve the outcomes of patients who had a stroke.
In this study, we found that old-old patients aged over 80 years were more likely to have worse prognoses under DAPT than that of younger patients, especially regarding composite vascular events, functional outcomes and mortality. The Second European Stroke Prevention Study trial found that, in all treatment groups, advanced age was associated with higher incidence of poor outcomes.26 Previous analyses of several large randomised controlled trials also demonstrated that stroke recurrence was higher in older patients regardless of the grouping.27–31 Our findings were consistent with previous studies, emphasising that age was one of the important independent predictors of poor outcomes in patients who had a stroke even with antiplatelet prevention.
This study also found that DAPT in elderly patients may lead to a relative increased risk of severe or moderate bleeding. Previous clinical trials have demonstrated that DAPT increased the risk of major bleeding and intracranial haemorrhage in old patients compared with younger patients.32 33 The Oxford Vascular Study also found that the risk of major bleeding increased steeply in patients treated with antiplatelet drugs aged over 75 years (HR 3.10, 95% CI 2.27 to 4.24; p<0.001), which was sustained during long-term follow-up.34 The possible causes for this increased risk comprise the following two points: First, owing to poor renal function in elderly patients, many drugs were difficult to excrete, therefore, drug plasma levels will increase, which may lead to higher bleeding complications.35 36 Second, it might be the effect of microbleeds. Cerebral microbleed load is certified to be associated with the increasing intracranial haemorrhage risk in patients treated with antithrombotic agents.37 38 Studies have also found that up to 6% of healthy elderly adults showed microbleeds on MRI.39 40 Additionally, several comorbidities in the elderly patients, such as hypertension and diabetes, were also closely associated with small vascular disease, which was visible as white matter hyperintensity in imaging, and may increase bleeding rates in these patients.
No differences were found in the efficacy and safety outcomes between the ticagrelor-aspirin group and the clopidogrel-aspirin group in old-old patients in our study, which differed from findings in younger patients. As previous studies have not analysed the effects of different DAPT regimens in elderly patients, our study suggests that genotype-guided DAPT might not be as effective in old-old patients as in younger ones, which provided clinical treatment clues for neurologists.
For elderly patients, it is important to consider the balance between the benefits and risks of DAPT when selecting the antiplatelet strategy. Currently, little is known whether the mechanism underlying the higher bleeding rate in elderly patients is owing to the old age or to the dual antiplatelet drugs, as lacking of clinical and scientific evidence. Old-old patients might not be simply contraindicated for DAPT considering the higher risks of composite vascular events and poor functional prognosis. Thus, more high-quality clinical trials involving elderly patients who had a stroke are needed to determine whether DAPT is the best option for acute and secondary prevention in elderly patients who had a stroke.
Our study has several limitations. First, all patients in the CHANCE-2 trial had CYP2C19 LOF alleles, and whether the findings can be generalised to patients without CYP2C19 LOF alleles is unclear. Second, the incidence of bleeding events was low in the CHANCE-2 trail, which may reduce the statistical power. Third, the sample size of patients >80 years was relatively small, older patients who had a stroke of larger cohort were needed in the future.
Conclusion
In conclusion, this study found that elderly patients aged ≥80 years with minor stroke or TIA had increased risks of composite vascular events, disabling stroke, severe or moderate bleeding and mortality within 90 days. Genotype-guided DAPT might not be as effective in old-old patients as in younger ones.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and the trial was approved by the ethics committee at Beijing Tiantan Hospital (IRB approval number: KY2019-035-02) and all participating centres. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We thank all study participants and their relatives as well as the members of the survey teams at the 202 centres of the CHANCE-2 study.
Footnotes
X @yilong
XZhang and YoW accepted full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish.
XZhang analyzed and interpreted the data and drafted the manuscript. JJ analyzed and interpreted the data. XX, HL, JLin, YiW, XZhao, ZL, YJ, LL, WC, XG, JLi and XH assisted to promote the project progress. AW and QX completed the statistical work. XM, SCJ, PMB and YoW conceived and designed the research.
Funding The study was supported by the Ministry of Science and Technology of the People’s Republic of China (MOST), Beijing Municipal Science & Technology Commission and Chinese Stroke Association (CSA), Beijing Municipal Science & Technology Commission, and grants from the National Science and Technology Major Project (2017ZX09304018), Capital's Funds for Health Improvement and Research (2020-1-2041), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5-029), and National Natural Science Foundation of China (81870905, 82101357, U20A20358). Salubris contributes ticagrelor, clopidogrel and its placebo at no cost and with no restrictions. Chongqing Jingyin Bioscience provides GMEX point-of-care genotyping system and technical supports for CHANCE-2 at no cost and with no restrictions.
Competing interests SCJ reports compensation from Everest CRO for data and safety monitoring services and compensation from AstraZeneca AB for consultant services. PMB reports grants from Alzheimer’s Society; compensation from DiaMedica for consultant services; compensation from Sanofi for consultant services; compensation from Phagenesis for consultant services; compensation from Moleac for consultant services; grants from British Heart Foundation; and grants from NIHR. YoW reports grants from Amgen; compensation from SANOFI-AVENTIS US for consultant services; grants from SANOFI-AVENTIS US; and grants from AstraZeneca. The other authors report no conflicts.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.