Abstract
Background and purpose Medullary infarction (MI) caused by spontaneous vertebral artery dissection (sVAD) is an important type of stroke. It is important to distinguish sVAD from other causes of stroke since the treatment strategies and prognosis were different between them. In this study, we aimed to explore the clinical and radiological features of MI in patients with acute MI caused by sVAD.
Methods Patients with acute MI caused by sVAD and non-sVAD in a single tertiary hospital were enrolled from 2010 to 2020. Epidemiologic, clinical and image features were collected and analysed. MI lesions were categorised into three levels rostrocaudally and four arterial groups: anteromedial, anterolateral, lateral and posterior.
Results A total of 128 patients with MI were enrolled with 47 cases of sVAD and 81 cases of non-sVAD. Patients with sVAD were younger than those with non-sVAD (med 44 years old vs 58 years old). The sVAD group was less likely to have hypertension (44.68% vs 67.90%; p=0.010) and diabetes (19.15% vs 45.69%; p=0.003), but more likely to have non-sudden onset (27.66% vs 9.87%, p=0.009), minor neck injury (19.15% vs 1.23%; p=0.001) and headache (46.81% vs 7.41%; p=0.000). Vertically, sVAD became more common in caudal medulla than in rostral medulla. Horizontally, the sVAD group was more likely to have lateral MI (91.48% vs 2.96%, p=0.000). In multivariable logistic regression analysis, age, non-sudden onset and headache were independently associated with sVAD with ORs of 0.935 (95% CI 0.892 to 0.981, p=0.006), 3.507 (95% CI 1.060 to 11.599, p=0.040) and 5.426 (95% CI 1.673 to 17.599, p=0.005).
Conclusion sVAD was not uncommon in patients with MI, especially in patients with lateral MI. Young patients with headache and non-sudden onset should remind clinician the possibility of sVAD.
Introductions
Medullary infarction (MI) is a rare but important type of ischaemic stroke. Increasing attention has been paid to the relationship between lesion topography of MI and different stroke mechanisms.1–3 Spontaneous vertebral artery dissection (sVAD) is an important cause of stroke mainly affecting young adults.4 5 Some patients with sVAD achieve partly or fully recovery of vertebral artery. The treatment strategies of sVAD are different from strokes of other causes, making it important to identify sVAD in clinical practice.6 7
However, underestimation of sVAD in clinical practice was not uncommon as it requires more extensive investigations regarding arterial vessel walls. So far, little has been known about clinical and radiological features of MI caused by sVAD. The purpose of this study is to investigate clinical and radiological features in patients with MI caused by sVAD and to analyse characteristics that distinguish sVAD from other conditions.
Methods
Study design
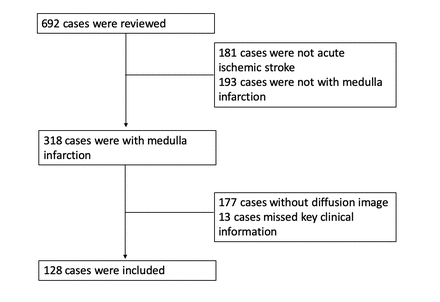
This is a hospital-based retrospective study. We performed a keyword search of ‘medullary infarction’ in the digital medical history system and picture archiving and communication system between May 2010 and May 2020 in a tertiary hospital. Inclusion criteria consisted of (1) acute neurological defect within 2 weeks, (2) acute infarction involving the medulla oblongata on diffusion weight imaging (DWI), (3) underwent at least one of the following vascular imagings: magnetic resonance angiography (MRA), CT angiography (CTA) or digital subtraction angiography (DSA). Exclusion criteria included: (1) patients after a major trauma,9 (2) patients younger than 18 years old. A total of 692 cases were screened and 128 patients were enrolled in this study (figure 1).
Flow chart for patient inclusion.
This study was approved by the Institutional Review Board of Huashan Hospital. The board waived the need for patient consent.
Definition
Stroke was defined as an acute disturbance of focal neurological function with symptoms lasting more than 24 hours. Non-sudden onset was defined as progressive development of symptoms/signs for longer than 1 day. Cardiovascular risk factors obtained from medical records were determined based on previous diagnoses and current medication as follows: diabetes (diagnosis or taken insulin or oral hypoglycaemic medication), hypertension (diagnosis or taken hypertension medication), coronary heart disease (diagnosis of angina pectoris, myocardial infarction, underwent percutaneous coronary intervention or coronary artery bypass grafting) and dyslipidaemia (diagnosis or total cholesterol ≥5.17 mmol/L or low dinsity lipoprotein (LDL) ≥3.36 mmol/L). Smoking was defined as currently smoking.
The diagnosis of sVAD was based on typical radiological characteristics (intimal flap, double lumen, dissecting aneurysm or luminal dilation plus stenosis) in at least one confirmatory angiographic examination including CTA, MRA and DSA. For an artery exhibiting non-specific stenosis or occlusion, sVAD will be diagnosed if intramural hematoma was identified in high-resolution MRI (HRMRI).8 Stroke mechanisms other than dissection were classified as follows: (1) large artery atherosclerosis (LAA) was determined by ≥50% stenosis or occlusion of the relevant vertebral artery, (2) cardiogenic embolism (CE) was diagnosed when there was emboligenic heart disease, (3) small vessel occlusion (SVO) was defined when there was infarction confined to a single perforator territory without evidence of LAD and CE, (4) the aetiology was considered as undetermined when: (1) two or more stroke causes were determined and (2) no mechanism could be determined after a thorough evaluation.3 9
Imaging interpretation
Images were reviewed on a Picture Archiving and Communication System workstation, and windowing was allowed. MI was identified on DWI and further evaluated on T2 and T1-weighted images for the determination of lesion locations by the consensus of two trained neurologists who had more than 3 years experience and with a focus on cerebral vascular diseases. In case of disagreement, images were further reviewed by an experienced radiologist. According to the location of infarction lesions, MI was divided into lateral MI and medial MI. Then the MI lesions were categorised into those involving the rostral, middle and caudal medulla oblongata.1 According to previous published templates for arterial territories, infarctions were classified into four groups (anteromedial, anterolateral, lateral and posterior).10 11 Vertebral perforative infarct referred to a small perforative arterial infarction in the medulla, including lateral MI and medial MI, without involvement of cerebellum and other areas of posterior circulation.12
All patients underwent at least one vessel lumen imaging, including 89 CTA, 13 MRA, 7 DSA, 10 both CTA and MRA, 3 both CTA and DSA, 2 both MRA and DSA, 4 CTA+MRA+DSA. HRMRI was performed in 52 patients. The scanning protocol of HRMRI including 3D-Time-of-Flight (TOF) sequence, Fast spin echo (FSE) sequence with 3D CUBE sequence (GE Medical Systems) or 3D SPACE sequence (SIEMENS Medical Systems). The vascular morphologies of sVADs were evaluated by another two neurologists who had over 5 years experience and with a focus on cerebral vascular diseases and further reviewed by an experienced neurologist in case of disagreement. The most proximal part (entry point) of a vascular dissection was defined as the site of artery dissection.8 Morphologies of sVAD are classified into five groups: (1) an intimal flap was defined as a layer crossing the arterial lumen, (2) double lumen was determined as blood flow that was divided into true plus false lumens, (3) luminal dilation was considered as dissecting aneurysm, (4) pearl-and-string sign was defined as aneurysmal dilation alternating with stenosis, (5) tapered steno-occlusion plus evidence of intramural haematoma (crescent-shaped, intermediate-to-high signal intensity thickening of the arterial wall according to haemorrhagic age).8 13–15
Statistical analysis
Statistical analysis was performed using SPSS V.26 (IBM Corporation). Categorical variables were reported as percentage and compared with the χ2 test or Fisher’s exact test. Continuous variables were reported as median and IQR and compared with the Mann-Whitney test. Clinical features and infarction lesion topographies between the sVAD and non-sVAD groups were compared using the χ2 test or the Mann-Whitney test according to the variable types. Variables with a p≤0.05 in the univariate analysis were entered into multivariate analysis model. Multivariate logistic regression analysis with enter method was conducted to determine factors independently associated with sVAD. P<0.05 was considered to be statistical significance. We also compared clinical and physical features in patients with lateral MI.
Results
Baseline characteristics
A total of 128 cases of MI were included in this study with 47 (36.72%) sVAD and 81 (63.28%) non-sVAD (table 1). There were more male patients than female patients in both groups. Patients with sVAD were younger and more likely to have history of minor neck injury (19.15% vs 1.23%; p=0.001) and headache (46.81% vs 7.41%; p=0.000) but less likely to have hypertension (44.68% vs 67.90%; p=0.010) and diabetes (19.15% vs 45.69%; p=0.003) (table 1).
Clinical features of MI caused by sVAD and non-sVAD
Clinical and lesion topography of MI caused by sVAD
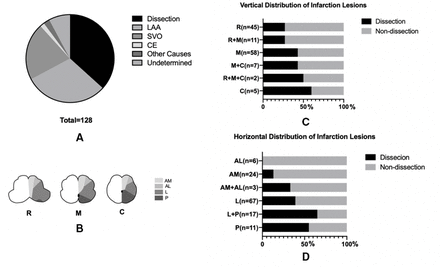
MI was unilateral in all patients with sVAD and 92.59% in the non-sVAD group. The sVAD group was more likely to have lateral MI than the non-sVAD group (91.48% vs 62.96%, p=0.000) (table 1). Vertically, sVAD became more common in caudal medulla than in rostral medulla. Horizontally, sVAD was more common in patients with lateral and posterior medullary lesions than those with anterior medullary lesions (figure 2).
Aetiologies of medullary infarction. Stroke aetiologies of medullary infarction (A); vertical and horizontal division of medulla (B); proportion of dissection in different lesion locations of medullary infarction vertically (C) and horizontally (D). AL, anterolateral; AM, anteromedial; C, caudal medulla; CE, cardiogenic embolism; L, lateral; LAA, large artery atherosclerosis; M, middle medulla; P, posterior; R, rostral medulla; SVO, small vessel occlusion.
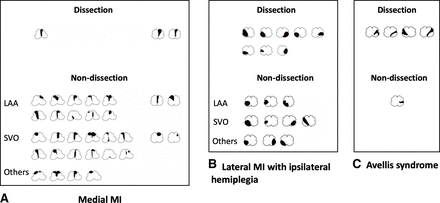
Most of medial MIs in our study were in rostral medulla oblongata. sVAD was uncommon in patients with medial MI. LAA and SVO were the two most frequent causes of medial MI (figure 3A). There were 94 patients with lateral MI (43 sVAD and 51 non-sVAD). Patients with lateral MI caused by sVAD more frequently presented with contralateral hemianaesthesia (sVAD 69.77% vs non-sVAD 47.06%, p=0.027) (table 2). Nineteen cases of patients with lateral MI displayed ipsilateral motor weakness, where eight cases (42.10%) were caused by sVAD (figure 3B). Five cases in our cohort had a possible diagnosis of Avellis syndrome, four of which were with sVAD (figure 3C).
Neurologic symptoms and signs of lateral MI
Example of lesion topography of medullary infarction Medial MI (A) ; Lateral MI with ipsilateral hemiplegia (B); Avellis syndrome (C). CE, cardiogenic embolism; LAA, large artery atherosclerosis; MI, medullary infarction; SVO, small vessel occlusion.
Vascular characteristics of sVAD and MI
We also analysed the relationship between the characteristics of sVAD and MI. As shown in table 3, all medial MIs and 79.07% of lateral MIs in the sVAD group had dissections involving the V4 segment of vertebral artery. 66.67% of medial MI and 81.39% of lateral MI in the sVAD group had vertebral artery stenosis (pearl-and-string sign or tapered steno-occlusion plus evidence of intramural haematoma).
Vascular characteristics of sVAD in medial MI and lateral MI
Association of clinical and lesion features with MI caused by sVAD
Age, non-sudden onset, hypertension, diabetes, minor neck injury, headache, lateral MI showed significant associations with sVAD in the univariate analysis. In multivariable logistic regression analysis, age, non-sudden onset and headache were independently associated with sVAD with ORs of 0.935 (95% CI 0.892 to 0.981, p=0.006), 3.507 (95% CI 1.060 to 11.599, p=0.040) and 5.426 (95% CI 1.673 to 17.599, p=0.005) (table 4).
Independent factors associated with MI caused by sVAD
Discussion
In this study, we compared clinical and lesion topography between patients with MI caused by sVAD and non-sVAD. Patients with sVAD tended to be younger with more frequently non-sudden onset and headache. Lateral MI was the most common type of MI caused by sVAD. Stenosis-occlusion was the most common angiography finding of sVAD in patients with MI.
sVAD was previously considered as a rare condition. The prevalence of sVAD was reported at 1.0 per 100 000 per year (95% CI 0.5 to 1.4) in Olmsted County, Minnesota.16 However, Inamasu reported that sVAD accounted for approximately 30% of cerebellar infarction in their cohort.17 In our cohort, 36.7% of MIs were caused by sVAD. Lateral MI was more common than medial MI in patients with sVAD, which was consistent with previous studies that the frequency of dissection in medial MI was not as high as in lateral MI.1–3 18 Previous studies also found that there were distinct stroke mechanisms between patients with medial and lateral MI. Artery dissection was the second most common cause in lateral MI, while SVO was more frequently observed in medial MI.3 18 19
Most patients with sVAD presented with vertebral artery stenosis or occlusion, which was also the most common angiography finding of sVAD in previous studies.20–23 There might be different mechanisms of infarction between sVAD and carotid artery dissection (CAD). Artery-to-artery embolism is the major mechanism of CAD while vertebral perforator infarct and small scattered infarct constituted most of the sVAD.12 15 24 In our cohort, involvement of V4 segment and vertebral perforator infarct were common in sVAD, suggesting that occlusion of vertebral perforator might be a major mechanism of MI caused by sVAD.
It should be noted that, in our cohort, more than 40% of patients with sVAD had hypertension and about 20% had diabetes. This result implied that even in patients with vascular risk factors, sVAD might be a possible cause of stroke, highlighting the importance of vascular wall imaging in clinical practice.
There were several limitations in our study. First, this was a retrospective study and clinical features were based on a review of medical records. Second, all patients included in this study were from Asian population, which might not well represent the whole picture of patients with MI. Third, during the 10-year study period, with the improvement in MRI technology, it is probable that some cases with sVAD would not be missed if the patient presented today. Finally, our study only demonstrated the characteristics between sVAD and non-sVAD, whether or not sVAD would increase the incidence of MI remains uncertain. More studies were needed to answer this question.
Conclusions
Dissection was not uncommon in patients with MI, especially in patients with lateral MI. Young patients with headache and non-sudden onset should remind clinician the possibility of sVAD.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author, Xiang Han, upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
The study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of Huashan Hospital. Because of the retrospective nature of the study, patient consent for inclusion was waived.
Acknowledgments
Thanks are due to Jiajia Yu for her help in English writing.
Footnotes
CY and ZZ are joint first authors.
WT and XH contributed equally.
Contributors Conception and design of study: XH, WT, QD. Acquisition of data: CY, ZZ, SL, YX, WY, XK, YL. Analysis and interpretation of data, drafting the manuscript: CY. Approving the final version of the paper: WT, ZZ, XH. XH is responsible for the overall content as guarantor. The guarantor accepts full responsibility for the finished work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding This work was supported by the Shanghai Shenkang Hospital Development Center (SHDC2020CR3067B), Shanghai Municipal Commission of Health and Family Planning (2018ZHYL0219) and Milstein medical Asian American partnership foundation (no award/grant number).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.