Abstract
Background and purpose The clinical significance of carbon dioxide combining power (CO2CP) in ischaemic cerebrovascular disease is not well established, and the role of CO2CP in the prognosis of acute ischaemic stroke (AIS) or transient ischaemic attack (TIA) has not been reported. The objective of the study was to investigate the associations between CO2CP and clinical outcomes in patients with AIS or TIA.
Methods Data were derived from the China National Stroke Registry III. Patients were classified into five groups by quintiles of CO2CP levels and three groups according to the normal range of CO2CP (23–29 mmol/L). Multivariable Cox and logistic regressions were adopted to explore the associations of CO2CP levels with all-cause death and poor functional outcomes (modified Rankin Scale (mRS) 3–6/2–6) at 3 months and 1 year.
Results Among 9531 patients included in the study, the median (IQR) CO2CP was 24.9 (23.0–27.0) mmol/L. After adjustment for potential confounders, patients in the first CO2CP quintile (21.1–23.3 mmol/L) had higher risk of all-cause death and poor functional outcomes (mRS score of 3–6/2–6) (HR or OR with 95% CI 2.37 (1.32 to 4.25), 1.49 (1.20 to 1.83) and 1.21 (1.03 to 1.42), respectively) compared with those in the fourth quintile. Similar results were found for outcomes at 1 year. Furthermore, all associations were also significant when CO2CP was <23 mmol/L compared with CO2CP of 23–29 mmol/L.
Conclusions Decreased CO2CP was associated with high risk of all-cause death and poor functional outcomes in patients with AIS or TIA.
Introduction
Carbon dioxide combining power (CO2CP) is a measure of alkali reserve and can help to diagnose the metabolic type of acidosis and alkalosis.1 2 The determination of CO2CP basically represents the amount of alkali reserves in the blood and determines whether an acid-base disturbance is present and if so to what degree. Decreased CO2CP has been demonstrated to be an indicator of metabolic acidosis in patients without respiratory disease.2
Previous studies have shown that metabolic acidosis is associated with insulin resistance,3 hypertension,4 inflammation,5 activation of the renin-angiotensin-aldosterone system6 and endothelial dysfunction,7 all of which can contribute to adverse clinical outcomes after stroke.3 8 Furthermore, acidosis is common and has been proven to be associated with poor outcomes in ischaemic stroke.9 Herein, it is logical to consider CO2CP as a risk factor for poor outcomes of stroke.
Clinical evidence has shown that decreased CO2CP is associated with advanced clinical stages of cancer and may predict worse outcomes of disease-free survival in patients with stage II/III colorectal cancer.1 Another study revealed that decreased CO2CP was an independent risk factor for the development of acute kidney injury and in-hospital mortality. However, the role of CO2CP in the prognosis of acute ischaemic stroke (AIS) or transient ischaemic attack (TIA) has not been examined to date. Therefore, in the present study, we sought to investigate the potential associations between CO2CP levels and adverse clinical outcomes in patients with AIS or TIA.
Methods
Study population
The data analysed in this study were obtained from the Third China National Stroke Registry (CNSR-III), which is a nationwide prospective registry including 201 hospitals of 22 provinces. The registry contains data of patients with AIS or TIA who presented to these hospitals from August 2015 to March 2018. A total of 15 166 participants with AIS or TIA at 7 days from symptom onset were enrolled in CNSR-III. The details, rationale and basic description of CNSR-III have been published previously.10
Data collection
Baseline data were prospectively collected using an electronic data capture system by face-to-face interviews following a standard data collection protocol that was developed by the steering committee, which included age, sex, body mass index (BMI; calculated as weight in kilograms divided by height in metres squared, kg/m2), medical history (hypertension, diabetes mellitus, dyslipidaemia, stroke or TIA, atrial fibrillation or flutter, peripheral vascular disease, heart failure, chronic obstructive pulmonary disease (COPD)), stroke type (ischemic stroke or TIA), aetiology classification of ischaemic stroke performed according to the TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria,11 current smoker, in-hospital medication (cholesterol-lowering agents, antihypertensive agents, hypoglycaemic agents, antiplatelet agents and anticoagulant agents), severity of stroke on admission (National Institutes of Health Stroke Scale, NIHSS),12 time from onset of symptoms to admission, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, fasting blood glucose, estimated glomerular filtration rate calculated using the creatinine-based Chronic Kidney Disease Epidemiological Collaboration equation13 and high-sensitivity C reactive protein (hs-CRP), and medication on discharge (cholesterol-lowering agents, antihypertensive agents, hypoglycaemic agents, antiplatelet agents and anticoagulant agents).
CO2CP testing
Fasting blood samples were collected at 24 hours after admission. The samples were placed into serum-separation tubes and EDTA anticoagulation blood collection tube and kept at room temperature. Afterwards, CO2CP was analysed by automated haematology analyser at 1 hour after sample collection at each research centre. All measurements were performed by laboratory personnel blinded to subjects’ clinical situations. CO2CP was analysed as a continuous variable and divided into quintiles.
Outcome assessment
Patients were followed up for clinical outcomes at 3 months and 1 year after symptom onset. They were interviewed face to face at 3 months and contacted over the telephone by trained research coordinators at 1 year. Data regarding all-cause death and poor functional outcome were collected by trained research coordinators who were blinded to subjects’ baseline characteristics. All-cause death was defined as death from any cause and confirmed by a death certification from the attended hospital or the local citizen registry. Poor functional outcome was defined as modified Rankin Scale (mRS) score ranging from 2 to 6 or from 3 to 6 at 3 months and 1 year.
Statistical analysis
Patients were classified into five groups by CO2CP quintiles. Continuous variables are described as median and IQR due to skewed distribution, and categorical variables are described as frequencies and percentages. Non-parametric Wilcoxon or Kruskal-Wallis test was used to compare group differences for continuous variables, and χ2 test or Fisher’s exact test was used for categorical variables.
We took the fourth quintile with the lowest incidence of outcomes as a reference and performed Cox proportional hazard models and logistic regression to explore the associations of CO2CP with all-cause death and poor functional outcomes, respectively. Because 201 hospitals participated in the study, the hospitals were treated as clusters in the model and the sandwich estimated was used to account for the correlations. Variables were adjusted in the multivariable analyses if associated with CO2CP in the univariate analysis with p<0.2. Adjusted HRs or ORs and their 95% CIs were calculated. We used three adjusted models. Model 1 was adjusted for age and gender; model 2 was further adjusted for BMI, medical history (IS, TIA, hyperlipidaemia and atrial fibrillation or flutter), stroke type, current smoker, antihypertensive agents, anticoagulant agents, NIHSS score and high-density lipoprotein cholesterol on admission; and model 3 was further adjusted for history of COPD, time from onset of symptoms to admission and hs-CRP on admission. Trend tests were performed in the regression models after the median CO2CP values of each quartile were entered into the model and treated as a continuous variable. Multivariate survival analysis was performed by the Kaplan-Meier method. In addition, we used restricted cubic splines to examine the shape of the association between CO2CP and outcomes with five knots (at the 5th, 25th, 50th, 75th and 95th percentiles); the reference point for CO2CP was the median of the reference group (the fourth quintile), and the HR/OR was adjusted for all confounding variables. Subgroup analyses were performed stratified by age (<65 and ≥65 years), gender, stroke subtype (IS and TIA), smoking status (yes and no) and NIHSS score (<4 and ≥4).
To further investigate the associations of CO2CP levels and outcomes, patients were classified into three groups (<23, 23–29 and ≥29 mmol/L) according to the normal range of CO2CP levels (23–29 mmol/L).2 Additionally, we also used C statistics, integrated discrimination (IDI) and net reclassification index (NRI) to evaluate the incremental predictive value of CO2CP beyond conventional risk factors.
All analyses were performed with SAS V.9.4 software. A two-tailed value of p<0.05 was considered statistically significant.
Results
Baseline characteristics
After exclusion of patients with missing data on CO2CP (n=5339) and mRS at 3 months or 1 year (n=296), 9531 patients were included in our analysis. Baseline comparison of the excluded and included patients is presented in online supplemental table S1.
Supplementary data
The baseline characteristics of included patients stratified into quintiles according to CO2CP are shown in table 1. The median (IQR) age of the patients was 62 (54–70) years, 6556 (68.79%) were men, and the median (IQR) CO2CP level was 24.9 (23.0–27.0) mmol/L. Compared with the rest of the quintiles, participants in the first quintile of CO2CP (21.1–23.3 mmol/L) had higher proportions of atrial fibrillation or flutter, more current smokers, more anticoagulant agent takers, a shorter time from onset of symptoms to admission and higher hs-CRP levels. On the other hand, participants in the fifth quintile (≥28.8 mmol/L) were more likely to be older, have higher proportions of dyslipidaemia and stroke, and have higher high-density lipoprotein cholesterol levels. In addition, patients in the fifth quintile were likely to have severe neurological deficits than those in the fourth quintile.
Baseline characteristics of included patients stratified into quintiles according to CO2CP
Associations of CO2CP with all-cause death and poor functional outcome
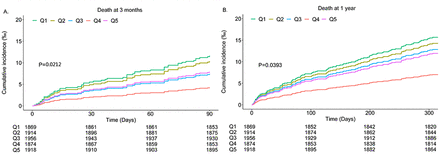
At 3-month assessment, 126 (1.32%) patients had died, 1288 (13.51) patients had poor functional outcome (mRS score 3–6) and 2484 (26.06%) patients had mRS score 2–6. At 1-year follow-up, 280 (2.94%) patients had died, 1235 (12.96%) patients had mRS score 3–6 and 2289 (24.02%) patients had mRS score 2–6 (table 2). Kaplan-Meier curves by quintiles of CO2CP showed that patients in the first quintile had a higher incidence of all-cause death both at 3 months and at 1 year (p<0.05) (figure 1A,B).
Kaplan-Meier curve of all-cause death incidence rate by quintiles of carbon dioxide combining power. (A) Death at 3 months and (B) death at 1 year. Adjusted for age, gender, body mass index, history of dyslipidaemia, stroke or transient ischaemic stroke, atrial fibrillation/flutter, stroke type, current smoker, antihypertensive agents, anticoagulant agents, National Institutes of Health Stroke Scale score, high-density lipoprotein cholesterol, history of chronic obstructive pulmonary disease, time from onset of symptoms to admission and high-sensitivity C reactive protein on admission.
Association of all-cause death and poor functional outcomes with quintiles of CO2CP
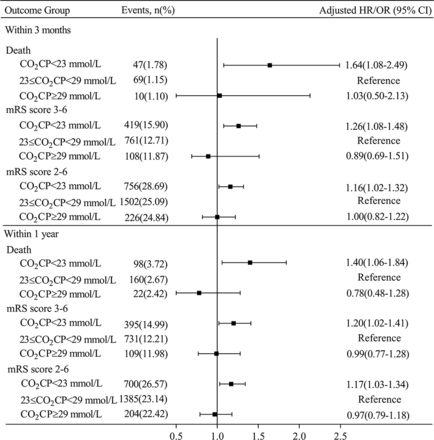
After adjustment for the above-mentioned confounding variables, patients in the first quintile CO2CP group were significantly associated with increased risk of all-cause death and poor functional outcome, compared with the fourth quintile of CO2CP taken as reference; the adjusted HR/OR (95% CI) was 2.37 (1.32 to 4.25), 1.49 (1.20 to 1.83) and 1.21 (1.03 to 1.42) for all-cause death, mRS score 3–6 and mRS score 2–6 at 3 months, respectively. The associations remained significant at 1 year (table 2).
All the associations were consistent in prespecified subgroups (all p for interactions >0.05; online supplemental table S2).
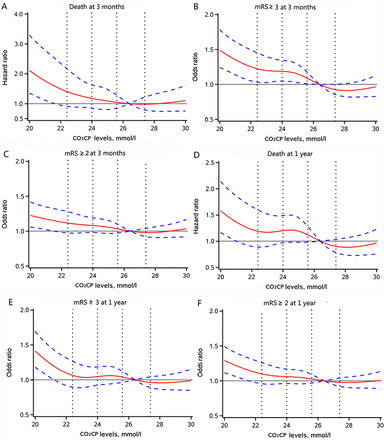
Multivariable-adjusted spline regression models showed that the relationships between CO2CP and adverse outcomes were non-linear. A lower CO2CP was associated with a higher risk of all-cause death at both 3-month and 1-year follow-up; as the CO2CP level increased, the HR of all-cause death declined steadily, showing a somewhat L-shaped associations. Furthermore, similar-shaped associations also existed for mRS score 2–6 and mRS score 3–6 at 3 months and 1 year (figure 2A–F).
Association of CO2CP levels with risk of all-cause death and poor functional outcome at 3 months and 1 year. (A–C) All-cause death, mRS score 3–6 and mRS score 2–6 at 3 months; (D–F) all-cause death, mRS score 3–6 and mRS score 2–6 at 1 year. Adjusted for age, gender, body mass index, history of dyslipidaemia, stroke or transient ischaemic stroke, atrial fibrillation/flutter, stroke type, current smoker, antihypertensive agents, anticoagulant agents, National Institutes of Health Stroke Scale score, high-density lipoprotein cholesterol, history of chronic obstructive pulmonary disease, time from onset of symptoms to admission and high-sensitivity C reactive protein on admission. CO2CP, carbon dioxide combining power; mRS, modified Rankin Scale.
In the analysis of the three groups, patients with CO2CP <23 mmol/L had higher proportion of atrial fibrillation or flutter and anticoagulant agent takers and higher hs-CRP levels compared with other groups. On the other hand, those with CO2CP ≥29 mmol/L were older and had higher proportion of stroke or TIA and less time from onset of symptoms to admission (online supplemental table S3). Furthermore, we found that patients with CO2CP <23 mmol/L had 64%, 26% and 16% higher risk of all-cause death and poor functional outcome (mRS score 3–6 and mRS score 2–6) compared with patients with normal CO2CP (23–29 mmol/L) at 3-month follow-up; the adjusted HR/OR (95% CI) was 1.64 (1.08 to 2.49), 1.26 (1.08 to 1.48) and 1.16 (1.02 to 1.32), respectively. A similar trend was observed at 1-year follow-up (figure 3). However, there were no significant associations between CO2CP and outcomes in patients with CO2CP ≥29 mmol/L.
Associations of CO2CP levels stratified into three groups with the risk of all-cause death and poor functional outcome at 3 months and 1 year. Adjusted for age, gender, body mass index, history of dyslipidaemia, stroke or transient ischaemic stroke, atrial fibrillation/flutter, stroke type, current smoker, antihypertensive agents, anticoagulant agents, National Institutes of Health Stroke Scale score, high-density lipoprotein cholesterol, history of chronic obstructive pulmonary disease, time from onset of symptoms to admission and high-sensitivity C reactive protein on admission. CO2CP, carbon dioxide combining power; mRS, modified Rankin Scale.
Incremental predictive value of CO2CP
We evaluated whether CO2CP would further increase the predictive value of conventional risk factors (online supplemental table S4). For death at 3 months as the outcome of interest, the C statistics by the conventional model did not significantly improve with the addition of CO2CP (from 0.841 to 0.843). However, the discriminatory power and risk reclassification appeared to be substantially better (IDI 0.50%, p=0.0254; continuous NRI=19.43%, p=0.0303). Similar results were found in poor functional outcomes and when the time point was set as 1 year.
Discussion
The major finding of the study was that decreased CO2CP was significantly associated with risk of all-cause death and poor functional outcome in patients with AIS and TIA at 3 months and 1 year. Further analysis showed that patients with CO2CP <23 mmol/L had high risk of adverse clinical outcomes, indicating that CO2CP of 23 mmol/L may be an important point to discriminate all-cause death and poor functional outcomes in patients with AIS or TIA in clinical practice.
In accordance with previous findings that decreased CO2CP is correlated with serious conditions and a poor disease prognosis,14–17 the findings of the present study showed that decreased CO2CP was significantly associated with all-cause death and poor functional outcomes after stroke. The clinical implications of CO2CP in the pathological conditions are mainly related to the acid-base disturbance, and studies have shown that the CO2CP level can aid in the diagnosis of the metabolic types of acidosis and alkalosis. Decreased CO2CP indicates insufficient alkali reserve and metabolic acidosis. Clinical data have shown that acidic pH is associated with an increased risk of mortality and unfavourable outcomes in patients with severe traumatic brain injury and ischaemic stroke.9 Moreover, an observational cohort study showed that metabolic acidosis was positively associated with a higher risk of ischaemic events and all-cause mortality in patients undergoing kidney transplantation.14 Results of several large cohort studies have further demonstrated that more severe metabolic acidosis is associated with higher mortality.15–17
Although most often decreased CO2CP signifies the presence of metabolic acidosis, it may also reflect a decline in the bicarbonate concentration as compensation for respiratory alkalosis, which is a disturbance in the acid-base balance due to alveolar hyperventilation and is the most common acid-base disturbance observed in patients who are critically ill. Respiratory alkalosis is considered benign by many clinicians, but it can also be associated with a significant increase in poor prognosis and mortality.18 Experimental studies have revealed that a risk in brain pH induced by decreased CO2CP impairs cortical GABAergic neurons, subsequently leading to vasoconstriction and deterioration of brain functions after transient ischaemia.19 20 Furthermore, respiratory alkalosis was reported to precipitate cardiac arrhythmias and angina, reduced myocardial oxygen extraction and increased mortality according to population-based studies.18 21 22 All of these changes can lead to adverse clinical outcomes.
There are several plausible explanations for the association between CO2CP and functional outcomes after AIS or TIA. First, metabolic acidosis induces insulin resistance, which can induce the production of proinflammatory cytokines in the brain after brain ischaemia.3 23 Second, metabolic acidosis is also associated with inflammation, oxidative stress and malnutrition, which may increase the risk of mortality.24 25 Third, acidosis is associated with toxic calcium influx into the cell and programmed cell death after ischaemic stroke.8 Because evidence regarding the associations between CO2CP and clinical outcomes in patients with stroke is limited, further investigation is needed to clarify the underlying mechanism.
Strengths and limitations
The strengths of the study include the use of a multicentre prospective registry with a large sample size, which resulted in sufficient statistical power. However, our study also had some limitations. First, this study only monitored baseline CO2CP levels and did not examine the dynamic changes in CO2CP, which may have provided more valuable information regarding the mechanisms underlying the associations. Second, heterogeneity of the equipment at different research centres may lead to biased estimates of results. However, this may have little impact because the hospitals were treated as clusters in the model and the sandwich estimated was used to account for the correlations in our study. Finally, some unmeasured or residual confounding effects may still exist due to the nature of the observational study.
Conclusions
Decreased CO2CP was associated with high risk of all-cause death and poor functional outcome in patients with AIS or TIA at 3 months and 1 year. These associations were significant when CO2CP level was <23 mmol/L. This finding underscores the importance of CO2CP in the prognosis of AIS or TIA in clinical practice.
Data availability statement
Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
Ethics statements
Ethics approval
The study was approved by the ethics committees of Beijing Tiantan Hospital and all other research centres according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all patients or legally authorised representatives before entering into the study.
Acknowledgments
We thank all participating hospitals, their physicians and nurses, CNSR III Steering Committee members and all the participants of the present study.
Footnotes
Contributors YW contributed to the conception and design of the study. AW contributed to manuscript drafting. HG, XT and YZ contributed to statistical analysis. XM and WL contributed to acquisition of data. HL contributed to critical revisions of the manuscript.
Funding This work was supported by the Young Elite Scientists Sponsorship Program by CAST (2018QNRC001), the Beijing Municipal Administration of Hospitals Incubating Program (PX2020021), the Beijing Municipal Science & Technology Commission (D171100003017002), and the National Science and Technology Major Project (2017ZX09304018).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.