Abstract
Background and purpose The low-field MRI is a promising tool to accurately diagnose strokes. We here report our study on the accuracy of a 0.23-Tesla (0.23-T) MRI using the haematoma enhanced inversion recovery (HEIR) sequence to detect acute ischaemic stroke (AIS) and intracerebral haemorrhage (ICH) within 24 hours of symptom onset.
Methods A novel HEIR sequence based on fluid-attenuated inversion recovery T1-weighted, with a scanning time of 1 min and 17 s, was developed using an ICH and AIS pig model on a 0.23-T MRI. Images of the pig model were obtained hourly for 24 hours in order to monitor value changes on T1/T2 and verify the differential diagnosis of AIS and ICH. Then, 30 patients with AIS and 30 patients with ICH with confirmed diagnoses by 3T-MRI/CT were included. Diagnostic criteria on a 0.23-T MRI for ICH was the hyperintensity signal on both the diffusion-weighted imaging (DWI) and HEIR sequence, while for AIS was the hyperintensity on DWI and isointensity on the HEIR sequence. Two blinded raters independently assessed the images obtained by the 0.23-T MRI for the presence of ICH/AIS.
Results In the pig model, setting the inversion time to 800 ms enabled clear differentiation of ICH from brain parenchymal tissue and AIS. In real patients, a correct 0.23-T MRI diagnosis of either an AIS or ICH was made in all 60 patients within 24 hours of symptom onset (100% overall accuracy). No adverse events occurred.
Conclusions The 0.23-T MRI may have the potential to differentiate cerebral haemorrhage from cerebral infarction with both speed and accuracy, making brain MRI scans easier, faster and cheaper. It might be possible to improve the screening imaging process for strokes in the emergency room. Further multicentre studies are needed to validate our findings.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Ruling out the presence of intracerebral haemorrhage (ICH) is a must when considering thrombolytic or antithrombotic therapies in patients who had a stroke. Current low-field MRI failed to achieve 100% accuracy to detect an ICH in a timely manner.
WHAT THIS STUDY ADDS
This experimental–clinical study developed a new approach on low field 0.23-Tesla (0.23-T) MRI incorporating a novel haematoma enhanced inversion recovery sequence and diffusion-weighted imaging sequence to detect both acute ischaemic and haemorrhagic strokes with great accuracy and speed.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Low field 0.23-T MRI scanner could be a new technology that makes obtaining brain MRI scans easier, faster and cheaper. It may revolutionise the screening imaging process for strokes in the emergency room.
Introduction
When a patient shows stroke-like symptoms, the treating physician must identify if the stroke is ischaemic or haemorrhagic. Ruling out the presence of intracerebral haemorrhage (ICH) is a must when considering thrombolytic therapies.1 The current practice is to use CT of the head since it is fast and available everywhere. However, conventional CT could miss an acute ischaemic stroke (AIS) lesion within the first few hours of symptom onset due to its limited sensitivity. MRI of the brain can provide a much better image of the brain, detect microhaemorrhages and rule out stroke mimics.2 However, conventional MRI systems (1.5–3-Tesla (T)) require long scanning time, good physical tolerance (no ventilators, vomiting or agitation), strict safety precautions (metals in body) and specialised technicians. It is not available in most emergency rooms or resource-limited settings.
Recent advancements in low-field MRI technology have allowed for shorter scanning time and accurate diagnosis of acute stroke. With an average examination time of 17 min and 51 s, a 0.064–T MRI can detect an ICH with an overall accuracy of 90.3% and a sensitivity of 80.4%.3 Recently, Mazurek et al4 included 189 examinations by a 0.064–T MRI. Their mean examination time was 34:15 min. This non-deep learning phase of scans had a sensitivity and specificity for ICH of 77.8% and 97.1%, respectively. Under the deep learning phase, these scans had improved to 96.6% and 99.3%, respectively.4 The limitations of current low-field MRI technology become evident as it fails to achieve 100% accuracy in detecting an ICH in a timely manner. As a result, low-field MRI still encounters significant challenges in replacing CT scans in emergency settings.
MRI of 0.23-T is a new technology.5 In this report, we aimed to observe signal changes in ICH and AIS within 24 hours using a 0.23–T MRI in pig models followed by the development of a novel haematoma enhanced inversion recovery (HEIR) sequence specifically designed for 0.23–T MRI. Transitioning to clinical settings, we compared its ability to detect an ICH/AIS between 0.23–T MRI (with HEIR and diffusion-weighted imaging (DWI) sequence) and conventional CT/MRI, with the goal of providing a preliminary indication of the potential deployment of low-field MRI in neurological emergencies.
Methods
Pig models of acute ischaemic/haemorrhagic stroke
In terms of grey and white matter composition and overall brain size, pig models are superior to small rodents for mimicking human brain physiology, allowing reliable tracking of brain damage evolution with multimodal imaging sequences.6–8 For the experimental ischaemic model,9 a Bama pig (male, aged 3 months, weight 12 kg) was anaesthetised with isoflurane at 3–4%; endothelin-1 33 µg dissolved in 150 µl of sterile water was injected at a rate of 50 µl/min into the brain through a cranial burr hole. In the experimental haemorrhagic stroke model,10 11 1.0 mL of autologous blood was infused over 10 min via a sterile plastic catheter through the cranial burr hole into the brain of a Bama pig (male, aged 4 months, weight 13 kg). After a 5-min pause, an additional 1.5 mL of blood was injected over 6 min. On completing the injection, bone wax was used to fill the burr hole and the skin incision was closed with sutures.
Pig models were positioned prone in a 0.23-T MRI (ACUTA Elfin, Ray Plus Medical Technology, Foshan, Guangdong, China) and imaged using an 8-channel head-neck coil. The 0.23-T MRI images were acquired every hour for 24 hours and included the following sequences (time to acquire): Fluid-attenuated inversion recovery T1-weighted (FLAIR T1) (repetition time (TR)/echo time (TE)=1326.0/24.4 ms, acquisition time=1 min and 32 s), FLAIR T2 (TR/TE=4380.0/107.9 ms, acquisition time=1 min and 32 s), T2WI (TR/TE=2500.0/150.4 ms, acquisition time=1 min and 33 s) and DWI (TR/TE=2598.2/100.3 ms, acquisition time=4 min and 22 s). All sequences were obtained in the axial plane. Further details regarding the sequence parameters are presented in table 1. The T1, T2 and relative proton density (PD) values were recorded.
Low-field portable MRI sequence details
The animals were under anaesthesia throughout the study to minimise any potential discomfort and eliminate motion artefact. This study adhered to established protocols and ethical standards for the care of laboratory animals. All animal experiments were approved by the Animal Use Subcommittee of a national stroke centre.
Participants
Approval for the study protocol was granted by our institutional ethical review board. Informed consent was obtained from all participants prior to 0.23-T MRI scans. This diagnostic study was conducted at a national emergency stroke centre from January 2024 to June 2024. We included 30 patients with AIS and 30 patients with ICHs. Their diagnosis was confirmed by CT (for ICH) and MRI (for AIS) of the brain. Conventional MRI and 0.23-T MRI examinations were performed after treatment while CT scans were conducted prior to treatment. All 60 patients underwent a 0.23-T MRI within 24 hours of their last known well-time.
Examination by a 0.23-T MRI integrated with HEIR sequence
The low-field 0.23-T MRI (ACUTA Elfin, Ray Plus Medical Technology, Foshan, Guangdong, China) was located in the emergency room. It is 160 cm (5.3 ft) in height, 120 cm (4 ft) in width and weighs 2400 kg (5300 Ib), offering a practical solution for spatial constraints (online supplemental figure 1). For safety, the critical boundary of the scanner has a 5 Gauss (0.5 mT) threshold that expands into a circle with a diameter of 130 cm, guaranteeing secure operation within its vicinity. The power source was 220V/10A complemented by a gradient peak amplitude of 25 mT/m and a gradient peak slew rate of 60 mT/m/ms, ensuring optimal performance in clinical settings. Patient comfort and accessibility were prioritised with the inclusion of a dedicated examination gurney capable of supporting a maximum weight of 150 kg (330Ib). The head coil, measuring 25.8 cm (11 inches) in height and 20 cm (8 inches) in width, facilitated precise imaging of cranial structures. It employs integrated shielding technology with both head and body shields and does not require any additional magnetic shielding measures.
Supplementary data
Diagnostic criteria in 0.23 T MRI for ICH were hyperintensity on both DWI and HEIR sequence, while for AIS were hyperintensity on DWI and isointensity on the HEIR sequence. The total examination time for 0.23-T MRI was 5 min and 51 s which included localiser (12 s), HEIR (1 min and 17 s) and DWI imaging (4 min and 22 s).
Outside the diagnostic criteria, all patients underwent T1WI and FLAIR sequences for further comparative analysis. The details regarding the sequence parameters are presented in table 1. Two blinded raters independently assessed the presence of AIS or ICH on 0.23-T MRI. The diagnostic accuracy of this new criterion in 0.23-T MRI for distinguishing between haemorrhage and ischaemia was compared with the final diagnoses determined by CT and MRI. The standard CT/MRI imaging was evaluated by a radiologist who was independent and blinded to the interpretation of the 0.23-T MRI.
Statistical analysis
Categorical data were presented as numbers with percentages and continuous variables were presented as mean±SD or median (IQR). The results of the 0.23-T MRI were compared with the corresponding CT/MRI by two independent neuroradiologists (JJ and QJ). Sensitivity and specificity were calculated. The intra-rater agreement for detection between the two imaging modalities of each rater was evaluated by Cohen’s κ statistics. A kappa value between 0.81 and 1.00 indicated almost perfect or excellent agreement. All statistical package analyses were performed with SPSS V.27.0 (SPSS, Chicago, Illinois, USA).
Results
Generation of HEIR sequence
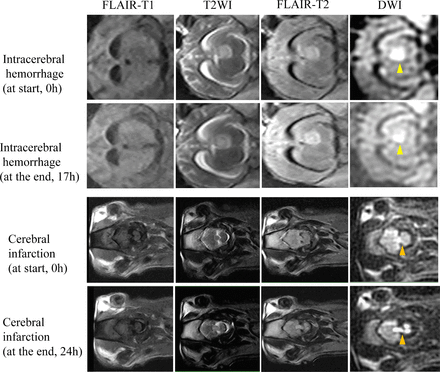
Based on the pig stroke models, we first depicted signal changes in ischaemic and haemorrhagic lesions on low-field MRI (figure 1). On 0.23-T MRI, the ICH exhibited high signal intensity on T2WI, FLAIR T2 and DWI within 17 hours with slightly low signal intensity on FLAIR T1 which did not change significantly over time. Unfortunately, the pig with ICH died at 17 hours of the scanning. For brain parenchyma, the T1 value was 420 ms, the T2 value was 105 ms and the PD value was 1.15; For ICH, the T1 value was 630 ms, the T2 value was 169 ms and the PD value was 1.45.
Sequential 0.23-T MRI of intracerebral haemorrhage and cerebral infarction in pig models. DWI, diffusion-weighted imaging; FLAIR, fluid attenuated inversion recovery; T1WI, T1 weighted image; T2WI, T2 weighted image.
For cerebral infarction, the T2WI, FLAIR T2 and DWI signals of cerebral ischaemia continued to increase and the FLAIR T1 signal continued to decrease within 24 hours. The cerebral infarction tissue may be composed of brain parenchyma (T1 value of 420 ms, T2 value of 105 ms, PD value decreased from 1.15 to 1.00), cerebrospinal fluid (T1 value of 4200 ms, T2 value of 2200 ms, PD value of 0.15) and oedema-like tissue (T1 value of 2200 ms, T2 value of 500 ms, PD value increased from 0.00 to 0.40).
The minimum TE/TR recovery time of 24 ms/1100 ms was selected for 0.23-T MRI. The online supplemental figure 2 depicted the changing signals of haemorrhagic, ischaemic and normal brain tissue based on the inversion time (TI) values on a 0.23-T MRI. It was found that with a TI of 800 ms (TE/TR of 24 ms/1100 ms), the ischaemic lesions and normal brain tissue showed similar signal intensities of 0.730, whereas the haemorrhagic lesions were distinguishable with a higher signal intensity of 0.818. The new HEIR sequence was developed from this differentiation. By suppressing the signals from both ischaemic lesions and normal brain tissue at a TI of 800 ms, the HEIR sequence visualised the signal of haemorrhagic lesions. Therefore, in the HEIR sequence designed for 0.23-T MRI, the haemorrhagic lesions exhibited high-intensity signals that could be clearly distinguished from ischaemic lesions and normal brain tissue.
Clinical application of HEIR sequence
Characteristics of enrolled patients are summarised in table 2. The median age of the patients was 60 years (IQR, 53.0–69.8 years) and 26.7% were women. The median time from symptom onset to CT was 5.9 hours (IQR 4.0–11.0 hours) and to 0.23-T MRI was 9.2 hours (IQR 5.1–12.2 hours). Among 30 cases of ICH, 15 were supratentorial (50.0%) and 6 were infratentorial (20.0%). Eight cases had intraventricular components and one was a subdural haematoma. The median diameter of the haemorrhagic lesions was 25.0 mm (IQR, 11.1 mm–30.3 mm) with approximately 10.0% (3 out of 30) being smaller than 5 mm. Among 30 cases of AIS, 17 (56.7%) were attributed to anterior circulation, 11 (36.7%) in the posterior circulation and 2 (6.7%) with both anterior and posterior circulations involved. The median diameter of the ischaemic lesions was 5.3 mm (IQR, 4.3 mm–13.9 mm) with approximately 50.0% (15 out of 30) being smaller than 5 mm.
Clinical characteristics of study population
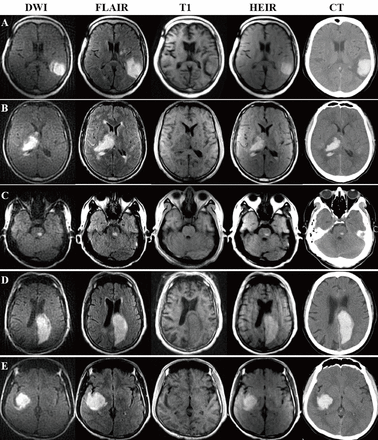
Table 3 shows the performance of 0.23-T MRI to diagnose ICH/AIS within 24 hours of symptom onset. Among the CT-detected 30 haemorrhages, the 0.23-T MRI identified all haemorrhagic lesions with both DWI and HEIR sequences showing hyperintensity. Among the conventional MRI-detected 30 cases of ischaemia, the 0.23-T MRI identified all ischaemic lesions with DWI showing hyperintensity and HEIR showing isointense signals. The cases of ICH and AIS on 0.23-T MRI were shown in figures 2 and 3, respectively.
The performance of 0.23-T MRI to diagnose ICH/AIS within 24 hours of symptom onset
Intracerebral haemorrhage on 0.23-T MRI and CT. Note: Diagnostic criteria in 0.23-T MRI for intracerebral haemorrhage are hyperintensities on both DWI and HEIR sequence. DWI, diffusion-weighted imaging; FLAIR, fluid attenuated inversion recovery; HEIR, haematoma enhanced inversion recovery; T1, T1-weighted image.
Acute ischaemic stroke on 0.23-T MRI and 3T-MRI. Note: Diagnostic criteria in 0.23-T MRI for acute ischaemic stroke are hyperintensities on DWI and isointensities on the HEIR sequence. DWI, diffusion-weighted imaging; FLAIR, fluid attenuated inversion recovery; HEIR, haematoma enhanced inversion recovery; T1, T1-weighted image.
In summary, a correct 0.23-T MRI diagnosis was made in 60 of the 60 patients who had an acute stroke (100% overall accuracy). The sensitivity of 0.23-T MRI in the detection of acute ischaemic/haemorrhagic stroke within 24 hours of symptom onset was 100% and specificity was 100%. The consensus among neuroradiologists in 0.23-T MRI assessments demonstrated complete agreement with a Cohen coefficient of 1. No adverse events occurred.
Discussion
This combined experimental–clinical study is the first to report on a new MRI sequence, HEIR (haematoma enhanced inversion recovery) that can distinguish between AIS and ICH within 24 hours of symptom onset by a 0.23-T MRI scanner. By integrating HEIR and DWI on a 0.23-T MRI, this study introduced a pioneering approach to early diagnosis of AIS or ICH with great accuracy (100%) in the fastest MRI scanning time (5 min and 51 s). Further studies are warranted to confirm these findings and to evaluate the potential of this approach in larger patient populations.
The imaging characteristics of cerebral haemorrhage and ischaemia on MRI are often intricate and variable, influenced by both the time postonset and the magnetic field strength. In cases of hyperacute cerebral haemorrhage, the increased deoxyhaemoglobin and paramagnetic substances in red blood cells lead to a shortening of the T2 relaxation time, typically presenting as isosignal or hyposignal on 1.5–3T MRI.12 13 However, since the magnetic susceptibility effect is directly related to the square of the magnetic field strength, hyperacute haemorrhage in low-field MRI is typically less influenced and may appear as a high signal.14 In cases of hyperacute cerebral ischaemia, MRI initially displays isosignal on T2-weighted images. Over time, due to blood-brain barrier damage, the ischaemic area gradually becomes hyperintense on T2-weighted images. Therefore, we hypothesised that low-field MRI could distinguish between hyperacute cerebral haemorrhage (high signal) and cerebral ischaemia (equal or low signal). The principle behind the HEIR sequence involves using an inversion recovery sequence with a carefully chosen inversion time (TI of 800 ms) to suppress the signal from ischaemic lesions.
For 1.5–3T MRI, standard imaging protocols for the detection of ICH typically include T2*-weighted gradient-recalled echo (T2*GRE) and susceptibility-weighted imaging (SWI) sequences, which have been proven to be more sensitive compared with non-contrast CT.15–19 However, the long scanning time of GRE/SWI is a drawback, particularly in emergency settings. Some studies have explored alternatives to substitute GRE/SWI such as DWI b0 to reduce examination time; however, it missed smaller haemorrhages and some parenchymal haematomas.20 21 Currently, achieving a balance between speed and accuracy in the detection of ICH using MRI has been a significant challenge. For low-field MRI, previous studies have explored various methods for differentiating ICH from AIS.3 4 Mazurek et al3 demonstrated that using T2WI and FLAIR sequences at 0.064-T MRI achieved an overall accuracy of 90.3% for detecting ICH. However, only 13 haemorrhages were in the hyperacute setting (within 24 hours of symptom onset) indicating potential limitations in applying these results to hyperacute bleeding conditions. Actually, on T1WI of 0.23-T MRI, our study showed hyperacute ICH as hyperintense, hypointense and isointense signals, while AIS presented as hypointense or isointense signals. On FLAIR of 0.23-T MRI, our study showed hyperacute ICH as hyperintense signals, while AIS presented as hyperintense or isointense signals. The variable signal intensities make it challenging to distinguish between cerebral infarction and cerebral haemorrhage using T1 and FLAIR imaging. Moreover, Mazurek et al4 further incorporated a deep learning reconstruction algorithm to 0.064-T MRI and found it had a sensitivity and specificity for ICH of 96.6% and 99.3%. Despite the improved accuracy, the mean examination time was 34:15 min for a 0.064-T MRI protocol that included prescan calibration, localiser, T1WI, T2WI, FLAIR and DWI. Although this protocol is valuable in resource-limited settings, it poses a challenge for patients with acute severe stroke and is not suitable for emergency situations.
The main strength of this combined experimental–clinical study is the development of a new approach on low field 0.23-T MRI incorporating a novel HEIR sequence and DWI to detect both AIS and ICH with great accuracy and speed. This new technology holds significant promise in assisting physicians treating strokes in the emergency room as well as those needing follow-up scans in the intensive care units or after interventional procedures. Moreover, the 0.23-T MRI operates on a standard wall outlet and is affordable to manufacture, maintain and operate. This low-cost MRI improves accessibility in low- and middle-income countries. Our study had limitations. It was based on a relatively small sample collected from a single institution which may limit the generalisability of the results. To ensure the reliability of our findings, more large investigations are needed at multiple centres worldwide.
Conclusion
The 0.23-T MRI may have the potential to differentiate cerebral haemorrhage from cerebral infarction with speed and accuracy, making brain MRI scans easier, faster and cheaper. It might be possible to improve the screening imaging process for strokes in the emergency room. Further multicentre studies are needed to validate our findings.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Approval for the study protocol was granted by institutional ethical review board in Tiantan hospital (KY2023-286-03). Participants gave informed consent to participate in the study before taking part.
Footnotes
XX and QJ contributed equally.
JJ and YW contributed equally.
Contributors Yongjun Wang, JJ and XX designed the study and supervised the overall project. ZW, Yihuai Wang, CZ and BX provided experimental samples to the manuscript. YS, HC, TL, ZZ and NW provided clinical samples to the manuscript. QJ did the statistical analysis. DW, QJ and XX wrote the manuscript. Yongjun Wang is the guarantor of this manuscript.
Funding This research received support from National Natural Science Foundation of China (grant no. 82271329; and grant no. 82471356).
Disclaimer Yihuai Wang, CZ and BX are employee of Ray Plus Medical Technology. The other authors have no conflicts of interest to be disclosed related to this article. The Tiantan Hospital holds a research agreement with Ray Plus Medical Technology who provided the 0.23 Tesla MRI.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.