Abstract
Background Although bypass surgery is an effective treatment for moyamoya vasculopathy (MMV), the incidence of postoperative complications is still high. This study aims to introduce a novel evaluating system based on individualised pathophysiology of MMV, and to assess its clinical significance.
Methods This multicentre, prospective study enrolled adult patients with MMV from Huashan Hospital, Fudan University and National Center for Neurological Disorders, China between March 2021 and February 2022. Multimodal neuroimages containing structural and functional information were used to evaluate personalised disease severity and fused to localise the surgical field, avoid invalid regions and propose alternative recipient arteries. The recipient artery was further selected intraoperatively by assessing regional haemodynamic and electrophysiological information. The preanastomosis and postanastomosis data were compared with assist with the postoperative management. Patients who received such tailored revascularisations were included in the novel group and the others were included in the traditional group. The 30-day surgical outcomes and intermediate long-term follow-up were compared.
Results Totally 375 patients (145 patients in the novel group and 230 patients in the traditional group) were included. The overall complication rate was significantly lower in the novel group (p˂0.001). In detail, both the rates of postoperative infarction (p=0.009) and hyperperfusion syndrome (p=0.010) were significantly lower. The functional outcomes trended to be more favourable in the novel group, though not significantly (p=0.260). Notably, the proportion of good functional status was higher in the novel group (p=0.009). Interestingly, the preoperative statuses of perfusion and metabolism around the bypass area were significantly correlated with the occurrence of postoperative complications (P˂0.0001).
Conclusions This novel evaluating system helps to identify appropriate surgical field and recipient arteries during bypass surgery for MMV to achieve better haemodynamic remodelling and pathophysiological improvement, which results in more favourable clinical outcomes.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Although the long-term effects of bypass surgery for moyamoya vasculopathy have been confirmed, the incidence of postoperative complications is high to result in poor clinical outcomes. The establishment of an individualised and pathogenesis-based evaluating system is necessary to improve the surgical efficiency.
WHAT THIS STUDY ADDS
The novel evaluating system based on individualised pathophysiology can provide objective information to localise appropriate surgical field and recipient artery in bypass surgery, which helps to decrease postoperative complications and results in more favourable clinical outcomes.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The standard operating procedure of individualised evaluation system for moyamoya vasculopathy is established and proved to be efficient. With this basis, revascularisation can achieve better haemodynamic remodelling and functional improvement.
Introduction
Surgical revascularisation is an efficient method for the treatment of moyamoya vasculopathy (MMV) that results in increased cerebral blood flow (CBF) and promotes cortical neovascularisation.1 2 Superficial temporal artery to middle cerebral artery (STA-MCA) bypass combining encephalo-dural-myo-synangiosis (EDMS) is a preferred revascularisation technique.2 3 Although the long-term effects of bypass surgery have been confirmed in large-sample studies,3–5 perioperative complications such as transient neurological deterioration due to cerebral hyperperfusion syndrome are common and result in poor clinical outcomes.4 6 7 However, the current concept of bypass surgery is just based on the clinical experience rather than objective evidence.8–11 Therefore, the establishment of an individualised and pathophysiology-based evaluating system is necessary to improve the surgical efficiency.9
Different selection of surgical field for bypass surgery may influence clinical outcomes.9 10 Yet, very few studies regarding selection criteria have been reported.12 According to our previous research,13 the progression of MMV includes a chain reaction of abnormal angioarchitecture, decreased cerebral perfusion, impaired brain metabolism and neurological dysfunction with a specific aetiology. With this basis, multimodal neuroimaging detecting these four factors should be performed preoperatively to evaluate the individual disease severity and to determine the surgical strategies.14 Both the target field and alternative recipient arteries can be determined with such system. In addition, invalid regions with metabolic deficits or intact perfusion can be identified.15
In general, there are multiple arteries located in the surgical field of bypass surgery, which can result in discrepancies in cerebral haemodynamic remodelling.10 Previous studies16 17 have reported that the selection of recipient artery based on indocyanine green (ICG) and electrocorticography (ECoG) are correlated with better clinical outcomes. ICG from the haemodynamic pattern and ECoG from the electrophysiological pattern can be conducted to select appropriate recipient arteries. Furthermore, these parameters can be constantly monitored during anastomosis and compared with their baseline values, which are useful for the postoperative management of the patient.16–18
Multimodal neuroimaging-guided neurosurgery was first reported in 1998 for tumour resecion.19 In the field of bypass surgery for MMV, such pathophysiology-based evaluating system can provide individualised information for surgical decision-making.1 20 21 This study aimed to assess its effectiveness and clinical significance for patients with MMV.
Methods
Patients
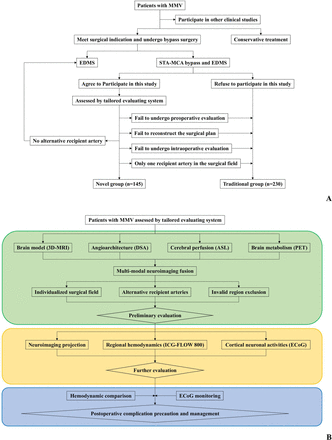
Adult patients diagnosed with MMV and received STA-MCA bypass combining EDMS at two medical centres of Huashan Hospital, Fudan University and National Center for Neurological Disorders, China between March 2021 and February 2022 were sequentially enrolled in this prospective study. All patients included in the novel group met the following criteria: (1) complete the multimodal evaluating flow chart; (2) have multiple recipient arteries in the surgical field; (3) exclude contraindications for magnetic resonance imaging (MRI) (such as metal implants or claustrophobia), digital subtraction angiography (DSA) (such as iodine contrast allergy or uncontrollable head movement) or ICG examination and (4) receive bypass surgery based on above individualised information.
Patients who did not meet the criteria of the novel group or received bypass surgery based on surgeon’s experience were included in the traditional group. All procedures in the two groups were performed by the same experienced neurosurgeons (YG and CG). The surgical outcomes within 30 days were recorded and compared between the two groups. Intermediate long-term neurological outcome was assessed using the modified Rankin scale (mRS).
Preoperative evaluation
Structural and functional neuroimaging
Brain model
MRI can be helpful to detect detailed structural changes in the grey matter volume. The three-dimensional T1-weighted MRI sequence (ie, 3D BRAVO sequence) was performed using a 3.0 Tesla MRI scanner (Signa HDxt, GE Healthcare, Milwaukee, Wisconsin, USA). The detailed 3D-T1 MRI parameters used in this study are provided in online supplemental materials.
Supplementary data
Cerebral angioarchitecture
DSA was performed to evaluate the hemispheric cerebrovascular architecture. Three-dimensional angiography of both sides of the common carotid artery and vertebral artery allows for the assessment of the cortical vessels’ distribution and compensation. The DSA parameters used in this study are provided in online supplemental materials.
Cerebral perfusion
Arterial spin labelling (ASL) is a non-invasive method used to quantify the cerebral perfusion status.22 All ASL images were acquired using a 3.0 Tesla MRI scanner (Signa HDxt, GE Healthcare, Milwaukee, Wisconsin, USA). Two postlabelling delays, which contained short ASL to reflect large vessel occlusion and delayed ASL to reflect small vessel compensation, were performed in this study.23 Abnormal areas with perfusion deficits were defined as those with decreased blood flow below the mean level of hemisphere. The detailed ASL parameters used in this study are provided in online supplemental materials.
Brain metabolism
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is the gold standard for assessing brain metabolism.24 The acquisition process of 18F-FDG PET was previously described.25 Raw images were reconstructed using a filtered back-projection algorithm with corrections for decay, normalisation, dead time, photon attenuation, scatter and random coincidences. The detailed PET parameters used in this study are provided in online supplemental materials.
Determination of the surgical field
Data fusion
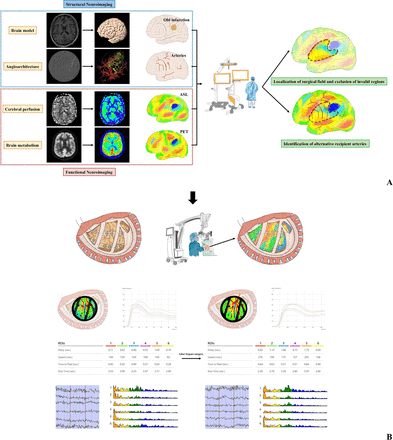
As shown in figure 1A, the multimodal neuroimaging data were transferred to the Elements software (Brainlab AG, Munich, Germany), in which all datasets were fused and superimposed using bony landmarks for reference. The accuracy of fusion was verified for visual inspection. Individualised brain model was reconstructed from 3D-T1 MRI and manual segmentation of cortical vessels was performed based on DSA. Both ASL and PET images were pseudocolourised and merged to brain model to reflect different situations of perfusion and metabolism on the cortical surface.
(A) Data processing flow chart for preoperative evaluation. (B) Data processing flow chart for intraoperative evaluation. Structural neuroImages including three-dimensional MRIs and digital subtraction angiography images are used to construct brain models and stereoscopic cerebral vessels. Arterial spin labelling (ASL) and positron emission tomography (PET) images are pseudocoloured to enhance the visualisation. Volumetric and angiographic images are fused to reflect old infarction areas and the distribution of the cortical arteries. Perfusion and metabolic images are overlayed using the preoperative navigation system, and the fused results are observed from multiple angles. with this basis, the hemispheric perfusion and metabolism statuses of different brain regions are compared with localise the surgical field and exclude invalid regions. In addition, alternative recipient arteries are determined. Both skin flap and bone windows are designed accordingly. Surgical field is further evaluated using indocyanine green (ICG) and electrocorticography (ECoG) monitoring. Multimodal neuroimaging was projected and overlaid with real-time surgical field under microscope and integrated navigation system using the augmented reality (AR) technique. The target recipient artery was identified with worse haemodynamic status using ICG-FLOW 800 and lower PSD in the high-frequency bands using ECoG. After bypass surgery, both haemodynamic and electrophysiological parameters are compared with those at baseline to determine the appropriate postoperative management strategy. ROIs, regions of interest.
Surgical plan determination
The perfusion and metabolic information were compared with localise the preliminary surgical field, which had relatively lower perfusion and metabolic statuses. Relative areas with old infarction or intact perfusion that cannot benefit from bypass were identified and excluded. Alternative recipient arteries were identified within the individualised surgical field. Both skin flaps and bone windows were designed accordingly.
Intraoperative evaluation
Structural and functional neuroimaging
Regional haemodynamic analysis
Our previous study confirmed that the application of ICG-FLOW 800 software (Zeiss Meditec, Oberkochen, Germany) is essential for further assessment of structural and haemodynamic information during bypass surgery.16 ICG videoangiography was performed for the previously-determined surgical field to distinguish the angioarchitecture of each artery. As the regions of interest (ROIs) were set at each artery branch in these areas, various haemodynamic parameters of alternative recipient arteries within the surgical field, including cerebral blood volume (CBV), CBF and time to peak (TTP) were calculated and compared intraoperatively. The details of ICG-FLOW 800 used in this study are provided in online supplemental materials.
Regional electrophysiological analysis
ECoG monitoring is useful for the assessment of neuronal activities, providing intraoperative verification of the regional function.17 ECoG was continuously recorded using BrainAmp MR PLUS (Brain Products, Gilching, Germany) to monitor neuronal electrical activity. Two 1×6 subdural electrode grids were placed in the middle frontal gyrus and superior temporal gyrus to cover the surgical field. The power spectral density (PSD) of each frequency band was calculated and the electrophysiological parameters of recipient arteries-located regions were compared. The PSD has been described previously15 and is expressed as follows:
(1) The PSD is the average of the Fourier transform magnitude squared over a large time interval.
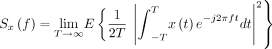
(2) The PSD is the Fourier transform of the autocorrelation function.
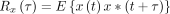
The power can be calculated from a random signal over a given frequency band as follows:
(1) Total Power in x(t):
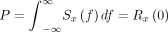
(2) Power in x(t) in the range f1–f2:
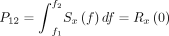
The detailed ECoG parameters used in this study are provided in online supplemental materials.
Determination of the recipient artery
The general process of revascularisation was detailed described in our previous studies.16 17 As shown in figure 1B, all the bypass-related information could be visible and verified for the determination of the recipient artery. Multimodal neuroimaging was projected and overlaid with real-time surgical field under microscope (Zeiss Meditec, Oberkochen, Germany) and integrated navigation system (Brainlab AG, Munich, Germany) using the augmented reality (AR) technique, which means that both perfusion and metabolic situations within the surgical field can be rechecked intraoperatively. Furthermore, the target recipient artery was identified with worse haemodynamic status using ICG-FLOW 800 and lower PSD in the high-frequency bands using ECoG.
The postanastomosis haemodynamic and electrophysiological parameters were compared with those at baseline to predict postoperative complications.16 17 For example, blood pressure management is of more importance for patients with high risks of hyperperfusion syndrome.18 Spick waves on the intraoperative ECoG indicate that antiepileptic medications are needed preventatively.7 The perfusion and metabolism around the anastomosis were evaluated postoperatively to identify possible postoperative complications.
Statistical analyses
Data were compared between the two groups using the unpaired t-test for demographic features and Bonferroni correction χ2 test or Mann-Whitney U test for clinical outcomes. Statistical significance was set at p<0.05. Database management and statistical analyses were performed using SPSS V.21.0 software (SPSS).
Results
Demographic features
A total of 375 adult patients with adequate clinical data were sequentially enrolled in this study, among which 148 patients were evaluated by multimodal neuroimaging (figure 2A). A flow chart of the tailored evaluating system is shown in figure 2B. No technique-related adverse events occurred and the overall success rate of the multimodal neuroimaging data fusion was 100% (148/148). The matching degree between the preoperative navigation scheme and the actual situation was 98.65% (146/148). Reconstruction of the data failed in two cases due to the insufficient diameter of the vessel and shifted position of the head during DSA. The success rate of the intraoperative AR projection was 99.32% (145/146), as one case had brain tissue collapse after cerebrospinal fluid release, which resulted in a severe deviation between target area and registration point. These three patients ultimately underwent traditional bypass. Therefore, a total of 145 patients were included in the novel group, while 230 patients were included in the traditional group (table 1). There were no differences in the age, sex ratio and vascular risk factors between the two groups.
(A) Flow chart and inclusion criteria of the study. (B) Standard operating procedure of the tailored evaluating system the standard operating procedure of the tailored evaluating system for MMV is divided into three parts: preoperative, intraoperative and postoperative. Preoperatively (green box), pathophysiology-based neuroimaging is fused so that the individualised surgical field and alternative recipient arteries are determined and invalid regions are excluded. Intraoperatively (yellow box), multimodal neuroimaging data are projected onto the surgical field and regional haemodynamic parameters as well as cortical neuron activities are assessed to verify the target recipient artery. Postoperatively (blue box), the changes in haemodynamic parameters and power spectral density are analysed to determine the appropriate postoperative management. 3D-MRI, three-dimensional magnetic resonance imaging; ASL, arterial spin labelling; DSA, digital subtraction angiography; ECoG, electrocorticography; EDMS, encephalo-dural-myo-synangiosis; ICG, indocyanine green; MMV, moyamoya vasculopathy; PET, positron emission tomography; STA-MCA, superficial temporal artery to middle cerebral artery.
Patient demographics and postoperative complications
Postoperative complications
The overall complication rate of the novel group was significantly lower than that of the traditional group (17.93% vs 34.34%; χ2=11.889,p˂0.001) (table 1). For subtypes of complication, the proportion of patients with cerebral infarction in the novel group was also significantly lower (1.38% vs 6.52%; χ2=6.846, p=0.009). The incidence of intracranial haemorrhage trended to be lower in the novel group, though not significantly (0.69% vs 2.17%; χ2=1.694, p=0.180). In addition, the proportion of patients with hyperperfusion syndrome was significantly lower in the novel group than in the traditional group (15.86% vs 24.35%; χ2=6.712, p=0.010).
Intermediate long-term follow-up
There were no differences of the follow-up periods between two groups (19.41±3.28 months vs 19.67±3.31 months; p=0.866). The overall neurological outcome trended to be more favourable in the novel group, though not significantly (z=1.127, p=0.260). Notably, good functional status (score of 0–1 on the mRS) at follow-up was observed in 92.41% of the patients in the novel group and in 83.04% of those in the traditional group (χ2=6.757, p=0.009) (table 1).
Mismatch phenomenon between ASL and PET-CT
The results of the perfusion and metabolism near the anastomosis area in the novel group indicated that these parameters differed among patients. It was hypothesised that the perfusion/metabolism mismatch may be related to individualised CBF reserves and collateral compensation (figure 3A).
(A) Perfusion/metabolism mismatch phenomenon. (B) Correlation with complication rate typical perfusion and metabolism status around anastomosis area: –/–, hypoperfusion and hypometabolism without obvious infarction; –/+, hypoperfusion and hypermetabolism with lacunar infarction; +/–, hyperperfusion and hypometabolism without obvious infarction; +/+, hyperperfusion and hypermetabolism without obvious infarction. hyper- is represented by ‘+’ while hypo- is represented by ‘–’ and the status of perfusion and metabolism is represented by */*. The total number of patients shows a downward trend with the change of perfusion and metabolism status, while the complication rate shows an upward trend. Patients with hypoperfusion and hyperpmetabolism had the lowest complication rate with statistical differences compared with other groups.
To determine the correlations between the mismatch phenomenon and surgical outcomes, the patients in the novel group were subdivided according to the perfusion/metabolism mismatch situation. In relation to the situation of the target region, hypoperfusion and hypometabolism were observed in 101 patients (69.66%); hyperperfusion and hypermetabolism were observed in seven patients (4.83%); hypoperfusion and hypermetabolism were observed in 22 patients (15.17%); and hyperperfusion and hypometabolism were observed in 15 patients (10.34%). The complication rates were significantly different among these subgroups (p˂0.001). Patients with both hypoperfusion and hypometabolism had the lowest complication rate (7.92%; Bonferroni correction, χ2=25.491, p˂0.0001) (figure 3B, table 2).
Mismatch phenomenon and correlation with complications
Illustrative case
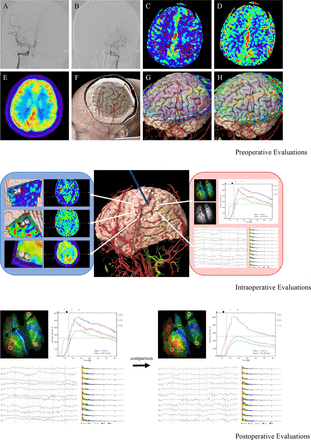
A 37-year-old man was admitted to our department with intermittent numbness of right upper limb. Severe stenosis of bilateral MCA with the formation of moyamoya vessels was demonstrated by DSA. Both hypoperfusion and hypometabolic changes were confirmed in the left temporal and parietal lobes by means of ASL and 18F-FDG PET. All these factors including cerebral angioarchitecture, perfusion and metabolism were assessed preoperatively for the individualise evaluation of the disease. As the spontaneous compensation from middle meningeal artery has been formed, personalised bone window was determined accordingly. Alternative recipient arteries were identified and the perfusion status in relative area could be compared. After the frontotemporal craniotomy, both perfusion and metabolic situations within the surgical field were visualised intraoperatively. Regional colour mapping was available with the application of ICG-FLOW 800 to distinguish recipient arteries and hypoperfusion areas. As the ROIs were set at the three branches of alternatives, the haemodynamic curves with CBV, CBF and TTP were calculated and compared. In addition, ECoG was continuously recorded and the electrophysiological parameters of recipient arteries-located regions were monitored. The selecting criteria of target recipient were based on the intraoperative haemodynamic and electrophysiological evaluation results. After anastomosis, postoperative ICG-FLOW 800 showed the good patency of the bypass and haemodynamic ameliorating effects were confirmed and ECoG also demonstrated the improvement of neuronal activity in relative region. Notably, spike waves and burst suppression phenomenon were found around the surgical area. With this basis, this patient was considered with high risk of hyperperfusion syndrome and epileptic attacks after the surgery. The sedative measures, strict blood pressure control, and sufficient doses of antiepileptic and neuroprotective medications were applied to avoid the complications. The patient was discharged 7 days without newly developed neurological deficits (figure 4).
Illustrative case of the novel evaluating system. For preoperative part, DSA demonstrated severe stenosis of bilateral middle cerebral artery with the formation of moyamoya vessels (A, B). ASL with two postlabelling delays showed hypoperfusion in the left temporal and parietal lobes (C, D). 18F-FDG PET also confirmed hypometabolism changes in the same area (E). Individualised bone window was designed to protect the blood supply from middle meningeal artery and alternative recipient arteries were identified (F). The perfusion status in relative area could be compared accordingly (G, H). For preoperative part, all the bypass-related information could be visible and verified for the determination of the recipient artery (blue box). ICG-FLOW 800 was performed and colour mapping was available. Haemodynamic curves and parameters could be calculated and electrophysiological parameters of recipient arteries-located regions were compared (red box). Number 1 of the recipients was selected as target artery. For postoperative part, the comparison of the parameters from ICG-FLOW 800 and ECoG could help to confirm the ameliorating effects and distinguish potential risk factors. ASL, arterial spin labelling; DSA, digital subtraction angiography; ECoG, electrocorticography; ICG-FLOW, indocyanine green; PET, positron emission tomography.
Discussion
Surgical revascularisation is gaining acceptance as one of the most useful therapies to improve CBF and reduce the risk of subsequent strokes for patients with MMV.1 2 Recent studies5 26 27 have confirmed the effectiveness of bypass surgery at the population level. However, postoperative complications often occur at the individual level and lead to a poor neurological prognosis.6 28 The incidence of complications ranges from 5% to 30%, which may be attributed to inherent differences of MMV and rigid surgical process.9 Previous studies9 10 12 16 have reported that the selection of recipient arteries may be a key factor for haemodynamic reconstruction and curative effects. However, the selection criteria are often based on the surgeon’s experience rather than objective information of the disease.8 Such situations urge the need for updated revascularisation concepts to improve patient outcomes.7 Therefore, surgical strategy should be determined according to the personalised status, which can result in precise structural and functional remodelling to reduce postoperative complications.9
Individualised evaluation based on pathophysiology
Based on the pathophysiological mechanism of MMV, several factors are needed to be considered in the preoperative evaluation.13 The multimodal neuroimaging technique allows surgeons to accurately assess the personalised disease severity from temporal and spatial perspectives and determine the appropriate treatment strategies.9 14 29 Hence, we creatively put forward the tailored evaluating system, which integrates individualised factors of MMV by combining multimodal neuroimaging for the preoperative determination of the surgical field, exclusion of invalid regions and selection of the alternative recipient arteries. With this basis, the modification of revascularisation focuses not only on structural reconstruction but also functional remodelling.27 30
During the intraoperative evaluating portion, the optional recipient vessels are further assessed using local haemodynamic analyses and neuroelectrophysiological monitoring.16 17 ICG-FLOW 800 provides detailed, real-time structural and haemodynamic information for each recipient arteries.31 ECoG is regarded as ‘the second microscope’ during surgical process, which helps to monitor and compare cortical electrophysiological activity in each frequency band.17 Cortical arteries with the weakest haemodynamic characteristics and the lowest electrical activity are identified so that the target recipient can be localised accordingly.32 Therefore, such tailored evaluating system allows for a comprehensive structural and functional assessment of the surgical field to achieve an evidence-based selection of the recipient artery.9
The establishment of a feasible prediction system for postoperative complications is also necessary. Current methods based on clinical symptoms and imaging changes are lack of timeliness and universality.33 According to previous researches,9 13 the axis of vascular anastomosis, blood flow improvement and changes in neuronal excitability plays a decisive role in bypass surgery. In the postoperative evaluating portion, the change rates of haemodynamic and electrophysiological status are analysed for precaution and management of postoperative complications.34 In this study, we took pathophysiological axis of MMV and mechanism of revascularisation into consideration to establish a personalised evaluating system. The ideal surgical fields and recipient artery could be localised preoperatively with objective evidence. Real-time parameters from haemodynamic and electrophysiological perspectives could also help to distinguish the high-risk patients and preventive measures for various complications could be developed in advance.
Ischemic penumbra and mismatch phenomenon of MMV
The evaluating standard for acute ischaemic stroke has been transformed from ‘time window’ to ‘tissue window’, which indicates that surgical indication should be based on mismatch volume between the penumbra and ischaemic core.35 Similarly, the evaluating focuses for chronic steno-occlusive cerebrovascular disease like MMV should also be transformed from the ‘perfusion window’ to the ‘metabolic window’.13 The application of PET has broadened the understanding of CBF and metabolic thresholds which are critical for the maintenance of brain function and morphology.36 Multiple studies37 38 have also confirmed the importance of the ischaemic penumbra (ie, brain regions with perfusion level below the functional threshold but above the structural threshold) in the evaluation of MMV. However, the clinical significance of cerebral glucose metabolic patterns in patients with MMV remains unclear.9 In this study, both perfusion and metabolism neuroimaging were compared with high-quality visualisation and spatial resolution. As the disease progresses and the degree of compensation varies in individual patients, the surgical area can be determined according to the location of the penumbra.
Interestingly, the perfusion and metabolism status around the target recipient had similar trends in most patients (74.49%), while a mismatch phenomenon was identified in 25.51% of patients in this study. Patients with hypoperfusion and hypometabolism have significantly decreased blood supply and weakened neuroactivity. Revascularisation for such population is necessary to improve the hemispheric blood supply and save the penumbra. For patients with hypoperfusion and hypermetabolism, misery perfusion can be explained as subclinical ischaemia with preserved neuronal viability.39 Revascularisation for such population aims to prevent decompensation. It is noteworthy that in some patients with extreme hypermetabolism, overcompensated neuronal viability may be influenced by the improvement of blood flow after bypass and result in abnormal neuronal electrical activity like epileptic attacks. Patients with hyperperfusion and hypometabolism represent a disease stage where neural function is decreased while compensatory vessels provide ‘luxury perfusion’.40 Revascularisation may influence the haemodynamic balance and result in complications. For patients with hyperperfusion and hypermetabolism, it may be not reasonable to perform revascularisation regarding high rate of complications.
Strengths and limitations
This study is not without limitations. First, such tailored evaluating system often requires more examinations, which is time-consuming and increases the medical costs. Second, the mechanism of the mismatch phenomenon and the reference weights for perfusion and metabolism information have not been clearly determined, which should be investigated in future studies. In addition, there may be no appropriate alternative recipient arteries in the target surgical fields and selection bias is also an issue that cannot be ignored. Therefore, the innovation of this system requires continuous optimisation.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and all procedures in this study were conducted in accordance with the Institutional Review Board of Huashan Hospital, Fudan University, China (approval number KY2019-329). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
This manuscript has been read and approved by all authors, who acknowledged due care in ensuring the integrity of the work. All authors have made substantial contributions to the design of the paper, collection, analysis and/or interpretation of the data, and most have contributed to the writing and intellectual content of the article.
Footnotes
Contributors Dr. YG and Dr. YM served as scientific advisors; Dr. YG and Dr. CG critically reviewed the study proposal; Dr. XZ and Dr. YL collected data and drafted the manuscript; Dr. JS, Dr. YjL and Dr. RF cared for study patients; Dr. DX and Dr. PG arranged the preoperative evaluations for the patients.
Funding This study was supported by the National Natural Science Foundation of China (grant number 82171313), Shanghai Hospital Development Center (grant number SHDC2022CRD032), Shanghai Municipal Science and Technology Commission Project (grant number 19DZ1930304), ZJLab, and Shanghai Zhou Liangfu Medical Development Foundation 'Brain Science and Brain Diseases Youth Innovation Program'.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.