Abstract
Background and objectives Prior evidence suggests that atrial fibrillation detected after stroke (AFDAS) is distinct from known atrial fibrillation (KAF), with particular clinical characteristics and impacts on outcomes in ischaemic stroke. However, the results remained inconsistent in ischaemic stroke, and the role of AFDAS in haemorrhagic stroke remains unclear. Therefore, we aimed to estimate the prevalence, risk factors and prognostic value of AFDAS in haemorrhagic stroke in comparison with ischaemic stroke.
Methods This was a multicentre cohort study. Patients who had an ischaemic and haemorrhagic stroke hospitalised in the Chinese Stroke Center Alliance hospitals were enrolled and classified as AFDAS, KAF or sinus rhythm (SR) based on heart rhythm. Univariate and multivariate logistic regression analyses were used to assess the prevalence, characteristics, risk factors and outcomes of AFDAS, KAF and SR in different stroke subtypes.
Results A total of 913 163 patients, including 818 799 with ischaemic stroke, 83 450 with intracerebral haemorrhage (ICH) and 10 914 with subarachnoid haemorrhage (SAH), were enrolled. AFDAS was the most common in ischaemic stroke. There were differences in the risk factor profile between stroke subtypes; older age is a common independent risk factor shared by ischaemic stroke (OR 1.06, 95% CI 1.06 to 1.06), ICH (OR 1.08, 95% CI 1.07 to 1.09) and SAH (OR 1.07, 95% CI 1.05 to 1.10). Similar to KAF, AFDAS was associated with an increased risk of in-hospital mortality compared with SR in both ischaemic stroke (OR 2.23, 95% CI 1.94 to 2.56) and ICH (OR 2.84, 95% CI 1.84 to 4.38).
Discussion There are differences in the prevalence, characteristics and risk factors for AFDAS and KAF in different stroke subtypes. AFDAS was associated with an increased risk of mortality compared with SR in both ischaemic stroke and ICH. Rhythm monitoring and risk factor modification after both ischaemic and haemorrhagic stroke are essential in clinical practice. More emphasis and appropriate treatment should be given to AFDAS.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Atrial fibrillation detected after stroke (AFDAS) was proposed as a novel specific clinical entity which may have distinctive clinical and prognostic characteristics. Studies of characteristics and prognostic value of AFDAS have yielded inconsistent conclusions in ischaemic stroke, and AFDAS in haemorrhagic stroke has rarely been studied.
WHAT THIS STUDY ADDS
Distinctive prevalence, characteristics and risk factor profiles of AFDAS were found in different stroke subtypes. In patients with ischaemic stroke and intracerebral haemorrhage, patients with AFDAS had a higher risk of in-hospital mortality than those with sinus rhythm, and there were no significant differences compared with patients with known atrial fibrillation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Rhythm monitoring, risk factor modification and AFDAS treatment are important in clinical practice after both ischaemic and haemorrhagic stroke.
Introduction
Multiple interactions occur among atrial fibrillation (AF) and cerebrovascular diseases. AF is a well-known risk factor for ischaemic stroke, and brain injury can in turn lead to arrhythmia.1 Determining whether AF occurring in patients is triggered by stroke or pre-existing and is the underlying cause of stroke is clinically significant but challenging. AF detected after stroke (AFDAS) was proposed as a new concept to characterise AF that was not known before but was newly detected after stroke, which is easy to diagnose and makes the classification of patients who had stroke with AF more convenient in clinical practice.2 AFDAS may comprise cardiogenic, neurogenic and mixed variants.3 In recent years, AFDAS has attracted considerable attention due to its therapeutic and prognostic significance. Identifying differences between AFDAS and known AF (KAF), which is defined as AF diagnosed before stroke onset, is important for understanding the mechanisms of AFDAS and characterising potential differences in the risk–benefit profile of oral anticoagulation.4 However, studies comparing AFDAS, KAF and sinus rhythm (SR) in ischaemic stroke have yielded inconsistent conclusions. Some studies have demonstrated that patients with AFDAS have a lower prevalence of cardiovascular comorbidities and a lower risk of recurrent ischaemic stroke than patients with KAF.4 5 Contrasting data have been reported by other studies.6 7
Moreover, prior studies on AFDAS have mainly focused on ischaemic stroke cases. Theoretically, haemorrhagic stroke can also trigger neuropathways of AFDAS, such as inflammation and autonomic dysregulation.8 9 Since AF is not a common aetiology of haemorrhagic stroke, AFDAS is less likely to be a pre-existing subclinical arrhythmia in haemorrhagic stroke than in ischaemic stroke. As such, investigating and comparing the characteristics of AFDAS and KAF in haemorrhagic stroke with ischaemic stroke may help to differentiate whether AFDAS is a manifestation of stroke-induced cardiac injury or an underlying cardiac condition.10 Additionally, understanding the prognostic value of AFDAS can help the development of prevention and therapeutic strategies in haemorrhagic stroke. However, the role of AFDAS in intracerebral haemorrhage (ICH) and subarachnoid haemorrhage (SAH) has rarely been studied.
To address the forgoing research gaps, we investigated the prevalence, clinical characteristics and risk factors for AFDAS and KAF in patients with haemorrhagic stroke in comparison with patients with ischaemic stroke. The effects of AFDAS on short-term outcomes in patients with different stroke subtypes were also assessed.
Methods
Study population
The present study was conducted using data from the Chinese Stroke Center Alliance (CSCA) database. The CSCA is a national, multicentre, hospital-based cohort that provides a unique platform to better understand a large cohort of patients hospitalised with stroke across 31 provinces in China. Details of the CSCA have been described previously.11
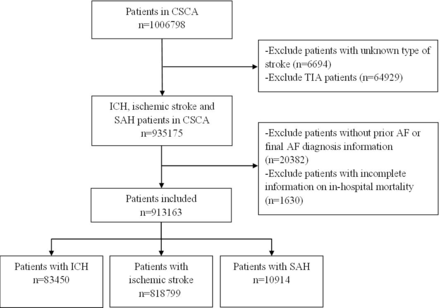
From August 2015 to July 2019, a total of 1 006 798 adult patients with stroke or transient ischaemic attack within 7 days of symptom onset from 1476 hospitals were consecutively recruited in the CSCA programme. After excluding 6694 patients with missing data on stroke type and 64 929 patients with transient ischaemic attack, a total of 935 175 patients with ischaemic stroke, ICH and SAH were enrolled initially. We further excluded 20 382 patients with missing data, including prior AF and final AF diagnosis information, and 1630 patients without primary outcome information. The remaining 913 163 patients were included in the final population, including 818 799 patients with ischaemic stroke, 83 450 with ICH and 10 914 with SAH (figure 1). When analysing the independent risk factors for AFDAS after different stroke subtypes, we further excluded 46 113 patients with KAF. A total of 867 050 patients were included in the analysis of independent risk factors for AFDAS in patients without prior AF.
Study flow diagram. AF, atrial fibrillation; CSCA, Chinese Stroke Center Alliance; ICH, intracerebral haemorrhage; SAH, subarachnoid haemorrhage: TIA, transient ischaemic attack.
Data collection and definition of covariates
The medical history and records of all the patients were reviewed for previously known AF by trained physicians. All patients underwent at least one ECG test during hospitalisation. ECG monitoring for 24 hours or longer was performed at the discretion of the treating physician. Based on their history of AF and results of ECG monitoring during hospitalisation, patients were classified into the following three groups: (1) SR (no prior AF and no AF detected during hospitalisation), (2) KAF (prior AF before stroke onset) and (3) AFDAS (no prior AF and AF diagnosed by ECG monitoring during hospitalisation). Data on clinical characteristics, medical history and laboratory findings were collected. Stroke severity was assessed by the Glasgow Coma Scale (GCS) score. Blood samples were collected on the second morning of admission at each subcentre, and biomarkers including fasting blood glucose, low-density lipoprotein cholesterol (LDL-C), glycosylated haemoglobin and creatinine were tested in all subcentres.
Outcomes
Outcomes assessed in this study included in-hospital mortality and in-hospital complications (myocardial infarction, epileptic seizure, deep venous thrombosis, rebleeding and cerebral infarction in patients with ICH and cerebral haemorrhage in patients who had an ischaemic stroke).
Statistical analysis
Continuous variables are expressed as the mean±SD for normally distributed data and median (IQR) for skewed distributed data. Comparisons between groups for continuous variables were tested by independent t-test or Kruskal-Wallis. Pearson’s Χ2 test was performed for comparison of categorical variables. Univariate and multivariable logistic regression analyses were used to determine the association between KAF, AFDAS and mortality in stroke subtypes. To determine the independent risk factors for AFDAS, we performed multivariable logistic regression analyses. Variables included were selected based on clinical importance and their statistical significance in the univariate analysis. Continuous variables were kept in their original forms in the multivariable models. A two-sided p value of <0.05 was considered to be statistically significant. All analyses were performed with SAS software V.9.4 (SAS Institute). The Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cohort studies were followed.
Results
Prevalence of AFDAS and KAF in different stroke subtypes
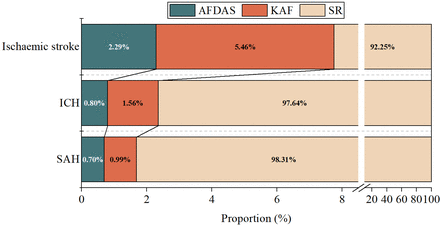
Of the 913 163 patients who had a stroke included, 83 450 patients had ICH, 818 799 had ischaemic stroke and 10 914 had SAH. The mean length of hospital stay was 16.5 days in patients with ICH, 11.6 days in patients with ischaemic stroke and 14.9 days in patients with SAH. During hospitalisation, among the 83 450 patients with ICH, 665 (0.80%) had AFDAS, 1305 (1.56%) had KAF and 81 480 (97.64%) had SR. Among the 818 799 patients who had an ischaemic stroke, 18 785 (2.29%) had AFDAS, 44 700 (5.46%) had KAF and 755 314 (92.25%) had SR. Among the 10 914 patients with SAH, 76 (0.70%) had AFDAS, 108 (0.99%) had KAF and 10 730 (98.31%) had SR. The prevalence of AFDAS, KAF and SR differed by stroke subtype (figure 2). Patients who had an ischaemic stroke had the highest rates of AFDAS and KAF, whereas patients with SAH had the lowest rates. The prevalence of AFDAS was higher in patients with ischaemic stroke than in those with ICH (2.29% vs 0.80%, p<0.0001) and SAH (2.29% vs 0.70%, p<0.0001). When compared with patients with SAH, patients with ICH had a higher prevalence of AFDAS (0.80% vs 0.70%, p<0.0001).
Prevalence of atrial fibrillation detected after stroke (AFDAS), atrial fibrillation known before stroke (KAF) and sinus rhythm (SR) in different stroke subtypes. Data are proportions. ICH, intracerebral haemorrhage; SAH, subarachnoid haemorrhage.
Clinical characteristics of AFDAS, KAF and SR in different stroke subtypes
When comparing baseline clinical characteristics between patients with AFDAS, KAF and SR in patients with ICH, compared with patients with KAF, those with AFDAS had a higher systolic blood pressure on admission and had a lower prevalence of prior hypertension, diabetes mellitus, dyslipidaemia, heart failure, myocardial infarction and stroke. Compared with patients with SR, those with AFDAS were older; more likely to be admitted to tertiary hospitals; had lower GCS scores on admission and higher CHA2DS2-VASC scores; and had a higher prevalence of prior myocardial infarction (online supplemental table 1).
Supplementary data
In patients who had an ischaemic stroke, compared with patients with KAF, those with AFDAS were older; more likely to be male; more likely to be admitted to tertiary hospitals; had higher systolic pressure on admission; had a lower prevalence of prior hypertension, diabetes, heart failure, myocardial infarction and stroke; and had a lower CHA2DS2-VASC score. Compared with patients with SR, those with AFDAS were older; more likely to be admitted to tertiary hospitals; had lower levels of systolic blood pressure; had higher CHA2DS2-VASC scores; had a higher prevalence of prior heart failure and myocardial infarction; and had a lower prevalence of prior hypertension, diabetes and stroke (online supplemental table 2).
In patients with SAH, compared with patients with KAF, those with AFDAS had a lower prevalence of prior stroke and had a lower CHA2DS2-VASC score. Compared with patients with SR, those with AFDAS were older, had higher CHA2DS2-VASC scores and had a higher prevalence of prior hypertension and diabetes (online supplemental table 3).
In-hospital complications and mortality
When comparing in-hospital complications between patients with AFDAS, KAF and SR among those with ICH, univariate analysis demonstrated that compared with patients with SR, patients with AFDAS had higher proportions of rebleeding, cerebral infarction, myocardial infarction, seizure and deep venous thrombosis (online supplemental table 1). In patients who had an ischaemic stroke, univariate analysis demonstrated that compared with patients with SR, patients with AFDAS had higher proportions of in-hospital complications, including myocardial infarction, seizure and deep venous thrombosis. Compared with patients with KAF, patients with AFDAS had lower rates of these in-hospital complications (online supplemental table 2). In patients with SAH, univariate analysis demonstrated that compared with patients with SR, patients with AFDAS had higher rates of myocardial function and seizures, but no significant difference was found between patients with AFDAS and KAF (online supplemental table 3).
The results from multivariable logistic regression analysis regarding in-hospital death in patients with ICH are presented in table 1. The fully adjusted models demonstrated that compared with patients with SR, both patients with AFDAS and KAF had a significantly higher rate of mortality (adjusted ORs 2.84; 95% CI 1.84 to 4.38 and 1.87; 95% CI 1.32 to 2.66, respectively). In contrast, the mortality rate in patients with AFDAS was not different from that of patients with KAF (adjusted ORs 1.51; 95% CI 0.88 to 2.62) in the fully adjusted model. In patients who had an ischaemic stroke, as shown in table 2, the fully adjusted models demonstrated that compared with patients with SR, both patients with AFDAS and KAF had a higher rate of mortality (adjusted ORs 2.23; 95% CI 1.94 to 2.56 and 2.23; 95% CI 2.02 to 2.46, respectively). In contrast, the mortality rate in patients with AFDAS was not different from that of patients with KAF (adjusted OR 1.00; 95% CI 0.86 to 1.16) in the fully adjusted model. In patients with SAH, as shown in table 3, the fully adjusted models showed that KAF (adjusted OR 2.75; 95% CI 1.12 to 6.75) but not AFDAS (adjusted OR 1.22; 95% CI 0.34 to 4.46) was associated with a higher mortality rate than SR. The mortality rate was not significantly different between patients with AFDAS and KAF (adjusted OR 0.45; 95% CI 0.09 to 2.10) in the fully adjusted model.
ORs (95% CIs) for mortality in patients with ICH according to KAF/AFDAS/SR
ORs (95% CIs) for mortality in patients who had an ischaemic stroke according to KAF/AFDAS/SR
ORs (95% CIs) for mortality in patients with SAH according to KAF/AFDAS/SR
Independent risk factors for AFDAS
In the multivariable logistic regression analysis, older age (p<0.0001), lower LDL-C levels (p=0.0423) and more severe stroke (p<0.0001) were independently associated with a higher risk of AFDAS in patients with ICH (online supplemental table 4). In patients who had an ischaemic stroke, older age (p<0.0001), female sex (p<0.0001), more severe stroke (p<0.0001), higher rates of prior heart failure (p<0.0001), myocardial infarction (p<0.0001) and use of anticoagulation agents (p<0.0001), lower rates of prior hypertension (p<0.0001), diabetes (p<0.0001), stroke (p<0.0001), current smoking (p<0.0001), and lower LDL-C level (p<0.0001) were independently associated with higher risk of AFDAS (online supplemental table 5). In patients with SAH, older age (p<0.0001) and prior hypertension (p=0.0404) were independent risk factors for AFDAS (online supplemental table 5).
Discussion
Several major findings emerged from this multicentre register-based cohort study. First, the prevalence of AFDAS was different in ischaemic stroke, ICH and SAH. Patients who had an ischaemic stroke had the highest rate of AFDAS, whereas the rate of AFDAS in patients with SAH was the lowest. Second, patients with AFDAS tended to have more in-hospital complications than patients with SR but fewer complications than patients with KAF. In patients with ischaemic stroke and ICH, patients with AFDAS had a higher risk of in-hospital mortality than those with SR, and there were no significant differences compared with patients with KAF. In patients with SAH, no significant associations were observed between AFDAS and in-hospital mortality.
Identifying the mechanisms of AFDAS is essential for secondary prevention strategies. Comparing the prevalence, clinical characteristics between AFDAS and KAF in different stroke types can help us better understand the mechanisms and determine therapeutic strategies of patients with AFDAS. There was evidence that AFDAS has a more benign profile, which indicates that patients with AFDAS may not need active monitoring and aggressive treatment as KAF.4 However, the results remain controversial to date.12–15 In addition, a recent study suggested that AFDAS can be separated into different subtypes according to detection time, including early and remote AFDAS, which is usually first detected beyond 3 months after stroke occurrence.16 Remote AFDAS is less likely to have any pathogenic role in the index stroke, while early AFDAS is more likely to be associated with the index stroke and may comprise a mix of cardiogenic and neurogenic phenotypes. All AFDAS in the current study was detected during hospitalisation within 1 month after index stroke, which represents early detected AFDAS. The results of the current study demonstrated that patients with AFDAS had a lower prevalence of cardiovascular risk factors and cardiac diseases than patients with KAF. However, in several previous studies conducted in Japan and Germany, patients with AFDAS and KAF were found to share common cardiovascular risk factors.17 18 Possible reasons for this discrepancy in findings may be due to differences in sample sizes and study populations. The results of several recent studies with larger populations conducted in America, Canada and China support our findings showing a lower frequency of cardiovascular risk factors in patients with AFDAS than in those with KAF.5–7 The results support the hypothesis that AFDAS is a different clinical entity with KAF and may be more likely to arise from neurogenic forces. On the other hand, the prevalence of AFDAS was significantly higher in patients with ischaemic stroke than in those with haemorrhagic stroke in the present study. This indicates that in addition to neurogenic forces, cardiogenic forces may also play an important role in AFDAS. A large proportion of AFDAS in patients with ischaemic stroke may pre-exist before stroke but remain undiagnosed until stroke. This kind of AFDAS bears a similar profile as KAF and is more likely to be observed in ischaemic stroke and be a causative agent. Of note, the prevalence of AFDAS was approximately 2.5% in patients who had an ischaemic stroke in previous studies,5 6 which is higher than that in our study. One possible reason is that ≥24 hours of monitoring was used in some previous studies. But not all patients who had a stroke in the current study underwent prolonged ECG monitoring. This means that the percentage of AFDAS is likely an underestimation since subclinical AF may have gone undetected. In a recent study, ECG-detected AFDAS was found to be associated with higher recurrent ischaemic stroke risk than prolonged cardiac monitoring-detected AFDAS.19 This indicated that there might be differences in characteristics and prognosis value between AFDAS detected by different methods. Future studies are needed to further confirm the clinical significance of AFDAS according to its different types.
Comparing the clinical outcomes of patients who had a stroke with AFDAS, KAF and SR can further help us determine the mechanisms and proper therapeutic strategies. However, previous studies targeted ischaemic stroke have yielded inconsistent results. A retrospective cohort study conducted in Canada reported no difference in the risk of recurrent stroke between patients with AFDAS and SR.5 On the other hand, two other cohort studies in America and China demonstrated that patients with AFDAS had an elevated risk of ischaemic stroke recurrence and mortality rate when compared with patients with SR.6 7 Another study conducted in Japan also demonstrated that stroke severity and in-hospital outcomes in patients with AFDAS did not differ from those with KAF.18 Similarly, in the current study with a larger sample size, we observed that similar to KAF, patients with AFDAS had a higher in-hospital mortality rate than those with SR, even after controlling for potential confounders. As such, active cardiac monitoring and treatments for AFDAS in patients with ischaemic stroke should still be given attention in the current clinical practice. Distinguishing neurogenic AFDAS from cardiogenic cases in the future is essential for individualised secondary prevention strategy determination.
Unlike ischaemic stroke, the prognostic value of AFDAS in patients who had a haemorrhagic stroke has rarely been investigated. A prior population-based registry study conducted in France showed that AF was not associated with an increased probability of early death in patients with ICH after adjusting for potential confounders.20 Notably, this study did not distinguish between KAF and AFDAS. The sample size in the previous study might also contribute to limited power to detect differences. In contrast, our results demonstrated that AFDAS in patients with ICH had a similar mortality rate when compared with patients with KAF and higher mortality when compared with patients with SR. This finding highlights that patients with ICH with AFDAS should also be regarded as high risk. Holistic cardiac surveillance and preventive strategies are warranted in these patients. Notably, study results and conclusions should be interpreted cautiously for the reason that not all patients who had a haemorrhagic stroke had undergone prolonged ECG monitoring in the current study. Some patients in the SR group might have AF but remained undetected. In future studies, uniform monitoring of cardiac rhythm after stroke onset will help us confirm the prognostic value of AFDAS and determine the optimal therapeutic strategy in patients with ICH.
Arrhythmia often occurs in the acute phase of SAH due to central sympathetic nerve system activation.1 21 Excessive catecholamine release after SAH leads to cardiomyocyte toxicity and cardiac system instability and induces the occurrence of AF.22 The results of the current study demonstrated that KAF, but not AFDAS, was associated with an increased risk of mortality in patients with SAH, which is inconsistent with a previous study, in which AF or atrial flutter occurred in 3.3% of patients with SAH and was associated with an increased risk of death.23 Of note, the rate of AFDAS among patients with SAH in our study was lower. One explanation is that the previous study did not differentiate KAF and AFDAS, and a substantial proportion of AF might pre-exist. Further studies with longer duration of follow-up are warranted to confirm our results and determine the role of AFDAS in patients with SAH.
We identified different risk factor profiles of AFDAS for different stroke subtypes; older age and a lower LDL-C level are common risk factors for AFDAS in patients free of prior AF shared by ischaemic stroke and haemorrhagic stroke. The same phenomenon has also been shown in the general population. Age is a well-known risk factor for AF due to increased atrial fibrosis.24 Higher LDL-C has also been found to have a protective effect against AF in the general population.25 The underlying mechanism may be that cholesterol, as the main component of cell membranes, can modulate the function of ion channels that are involved in the initiation of AF.26 Of note, since ECG monitoring for 24 hours or longer was performed at the discretion of the treating physician, patients with older age and more other risk factors might be more likely to receive prolonged cardiac monitoring after stroke. Therefore, the risk factors for AFDAS detected in the current study may not be completely representative of new-onset AF after stroke. Future studies with more uniform monitoring methods are needed to confirm the risk factors for AFDAS and understand the pathophysiological pathways to prevent AFDAS from occurring and perpetuating atrial remodelling.
This study draws strength from the large, comprehensive and validated inpatient registry data. However, several limitations should be considered. First, the diagnosis of AFDAS was based on ECGs or 24-hour Holter monitoring, which may cause a detection bias, especially in patients who had no ischaemic stroke and in those with fewer risk factors for AF. Despite this, our data from the large national database are a reflection of AFDAS detection and its prognostic value in a current real-world clinical setting. Future studies with uniform long-term cardiac monitoring after stroke are needed to confirm our findings. Second, there was a lack of information regarding the location and size of the lesions, which may have biased the results. The characteristics of lesions and their impact on outcomes should be considered in future studies. Third, we could not distinguish neurogenic cases from cardiogenic cases in AFDAS to assess their characteristics and impact on outcomes. Identifying the specific pathophysiology of AFDAS is essential and should be established in experimental models. Finally, the study was conducted among a homogeneous population in China, and whether these results are generalisable to other non-Asian populations remains to be investigated.
Taken together, our results suggested that in ischaemic stroke, although AFDAS cases have less abnormal cardiac substrate, cardiac mechanisms might still be important triggers. The relationship between AFDAS and increased risk of mortality highlights the importance of rhythm monitoring, risk factor modification and appropriate treatment for AFDAS after both ischaemic and haemorrhagic stroke. More targeted studies are needed to better address the pathophysiology of AFDAS and identify specific biomarkers to distinguish neurogenic AFDAS from cardiogenic AFDAS. Additionally, randomised clinical trials are needed to determine the most appropriate secondary strategies for patients who had a stroke with AFDAS.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the Ethics Committee of Beijing Tiantan Hospital. Participating hospitals received either healthcare quality assessment and research approval to collect data in the CSCA project without requiring individual patient informed consent under the common rule or a waiver of authorisation and exemption from subsequent review by their Institutional Review Board.
Footnotes
X @hqgu
YW and XZ contributed equally.
Contributors Guarantor who responsible for the overall content—XZ. Conception and design of the study—JG, ZL, YL and JL. Acquisition and analysis of the data—H-QG, K-XY, JG, DW, JJ and JZ. Drafting of the manuscript and preparation of tables—JG and ZL. YW and XZ accept full responsibility for the work and the conduct of the study, had access to the data and controlled the decision to publish.
Funding This study was supported by grants from the National Key R&D Program of China (2022ZD0118005), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5-029), Beijing Municipal Committee of Science and Technology (Z201100005620010), Beijing Hospitals Authority Innovation Studio of Young Staff Funding Support (202112) and Ministry of Finance of the People’s Republic of China (issued by Finance and Social Security (2015) Document No. 82; (2016) Document No. 50; (2017) Document No. 72; (2018) Document No. 48; (2019) Document No. 77; (2020) Document No. 75; (2021) Document No. 84, Ministry of Finance).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.