Abstract
Objective The long-term postoperative language outcomes for brain arteriovenous malformations (bAVMs) have not been well characterised. With fibres scattered in the Broca’s, Wernicke’s and Geschwind’s area, the arcuate fasciculus (AF) is considered as a crucial structure of language function. This study aimed to observe the language outcomes, determine the risk factors and construct a grading system for long-term postoperative language deficits (LDs) in patients with bAVMs involving the AF (AF-bAVMs).
Methods We retrospectively reviewed 135 patients with AF-bAVMs. Based on the course of the AF and our clinical experience, three boundary lines were drawn to divide the AF into segments I, II, III and IV in spatial order from the frontal lobe to the temporal lobe. Surgery-related LD evaluations were performed 1 week (short term) and at the last follow-up (long term) after surgery. Finally, based on multivariable logistic regression analysis, a grading system was constructed to predict long-term postoperative LD. The predictive accuracy was assessed using the area under the receiver operating characteristic curve (AUC).
Results Sixty-two (45.9%) patients experienced short-term postoperative LD. After a mean follow-up of 50.2±24.9 months, long-term LD was found in 14 (10.4%) patients. Nidus size (p=0.007), LD history (p=0.009) and segment II involvement (p=0.030) were independent risk factors for short-term LD. Furthermore, segment II involvement (p=0.002), anterior choroidal artery (AChA) feeding (p=0.001), patient age (p=0.023) and LD history (p=0.001) were independent risk factors for long-term LD. A grading system was developed by combining the risk factors for long-term LD; its predictive accuracy was 0.921.
Conclusions The involvement of the trunk of the AF between Broca’s area and the inferior parietal lobule, a nidus supplied by the AChA, older patient age and history of LD were associated with long-term postoperative LD. The grading system combining these factors demonstrated favourable predictive accuracy for long-term language outcomes.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Surgical treatment of brain arteriovenous malformations involving the arcuate fasciculus (AF-bAVMs) is challenging because invasive damage may result in language deficit (LD). However, the long-term postoperative language outcomes for AF-bAVMs have not been well characterised, and no specific grading system for the treatment of AF-bAVMs is available.
WHAT THIS STUDY ADDS
We first described the language outcomes in a cohort of 135 patients with AF-bAVMs after surgery with a mean follow-up of 50.2 months and identified the risk factors for short-term and long-term postoperative LD.
We proposed a new method to segment the AF using diffusion tensor imaging tractography and demonstrated that the involvement of the segmentation located in the fronto-occipital junction had the highest risk of postoperative LD.
We constructed a grading system to predict long-term postoperative LD of AF-bAVMs with satisfactory predictive performance.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
We identified the long-term postoperative language outcomes for AF-bAVMs and provided a grading system for selecting surgical candidates in patients with AF-bAVMs.
Introduction
With fibres scattered in the Broca’s, Wernicke’s and Geschwind’s area, the arcuate fasciculus (AF) is considered a crucial structure of language function.1 Surgical treatment of brain arteriovenous malformations (bAVMs) involving the AF (AF-bAVMs) could cause a high incidence of postoperative language deficits (LDs).2 Previous studies have shown that some patients with postoperative LD have favourable recovery potential.3 Additionally, bAVM is often diagnosed in youth,4 and it is a benign disease that rarely recurs after surgical resection,5 6 which means that patients have a long postoperative survival time. Therefore, evaluating the postoperative long-term language function of patients with AF-bAVMs becomes a top priority to identify the real risk factors for postoperative LD. Given previous literature regarding AF-bAVMs is limited to a follow-up of less than 2 years,2 3 there is an urgent need to evaluate the long-term outcomes in patients with AF-bAVM resection in a longer follow-up.
Complete resection has always been required for bAVMs,7 8 which makes it difficult to protect the invaded language structure by partial resection as with gliomas.9 Careful case selection is necessary to minimise postoperative neurological deficits in patients with bAVMs. However, no specific grading system for the treatment of AF-bAVMs is available. According to our clinical practice, we hypothesised that bAVMs located in different segments of AF may have different surgical outcomes, which might guide the selection of optimal surgical candidates. To date, few studies compared the language outcomes following the resection of bAVMs among different segments of the AF. A grading system with the consideration of the involvement of AF segmentation for pre-surgically evaluating the postoperative LD, especially for long-term LD, is needed.
In the past few years, favourable feasibility and accuracy of diffusion tensor imaging (DTI) tractography in visualising white matter have been confirmed by several studies.10–12 In our study, we described the incidence and identified the risk factors for short-term and long-term postoperative LD in a cohort of 135 patients with a mean postoperative follow-up of 50.2 months. To evaluate the influence of different AF parts, the AF was divided into four segments using DTI tractography based on the characteristics of the AF course and our clinical experience. Finally, a grading system was constructed based on the AF segmentation for the prediction of long-term LD, which may help clinicians select surgical candidates.
Methods and materials
Study population
We retrospectively reviewed 135 patients with AF-bAVMs who underwent microsurgery from our bAVM database of two prospective clinical trials (ClinicalTrials.gov Identifier: NCT01758211 and NCT02868008) between January 2013 and May 2020. With a further follow-up, sixty-eight (50%) patients included in our previous study were also recruited in the present study. AF-bAVMs were defined as bAVMs located in the Broca’s area, Wernicke’s area, Geschwind’s area and other areas involving the AF.2 13
Neuroimaging
A brain MRI series were obtained for all patients using a 3.0-T MR scanner (SIEMENS Trio). Detailed protocols for obtaining sagittal T1-weighted anatomical MRIs, DTI and time-of-flight magnetic resonance angiography (TOF-MRA) were described in the online supplemental material 1.
Supplementary data
Neuroimaging data analyses
To visualise the AF, we performed DTI tractography on the iPlan cranial 3.0 workstation (Brainlab). Detailed protocol for AF tracking was described in the online supplemental material 1.
AF classifications
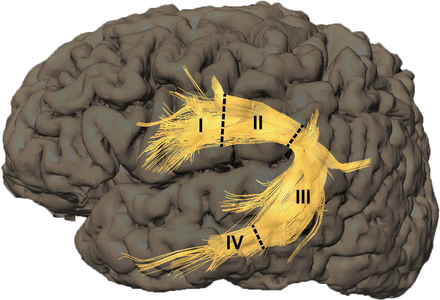
The AF is a white matter fibre tract that connects Broca’s area, the inferior parietal lobule and Wernicke’s area via a projection that arches around the Sylvian fissure. The fibres are scattered in Broca’s area, Wernicke’s area and the inferior parietal lobule, while between Broca’s area and the inferior parietal lobule, the fibre tracts converge into a trunk.1 14–16 According to the characteristics of the AF course, we delineated three boundary lines: the first line was located where the last fibre of the AF that scattered in Broca’s area (Brodmann Area (BA) 44, BA 45) merged into the trunk of the AF; the second line was located where the first branch of the AF extended from the converged trunk to the inferior parietal lobule (BA 39, BA 40); and the third line was located at the anterior border of Wernicke’s area (BA 22). According to the criteria, the AF was divided into four segments with these three boundary lines, and segments I, II, III and IV were arranged according to the direction from the frontal lobe to the temporal lobe (figure 1). The nidus location was identified using TOF-MRA and DTI tractography, and involvement of the AF was documented if the shortest distance from a nidus to the AF was less than 5 mm.2 10 17 Niduses involving more than one AF segment were counted repeatedly.
Based on the characteristics of the AF course and our clinical experience, we defined three boundary lines (dashed lines). The first line was located where the last fibre of the AF in Broca’s area merged into the trunk of the AF. The second line was located where the first branch of AF extended from the trunk to the inferior parietal lobule. The third line was located at the anterior border of Wernicke’s area. These three boundary lines divided the AF into four segments, which were designated I, II, III and IV in spatial order from the frontal lobe to the temporal lobe. AF, arcuate fasciculus.
Two neurosurgeons (YJ and JW) performed the judgement of AF segmentation involvement, and discrepancies were resolved by a senior neurosurgeon (YC). By calculating the inert-observer ratio (kappa), good reproducibility was found for the identification of AF segmentation involvement (Cohen’s κ=0.937, 0.856, 0.940, and 0.907 for the detection of segmentation I, II, III, and IV involvement, respectively) in all patients (detailed information was provided in the online supplemental material 1).
Previous studies have indicated that anterior choroidal artery (AChA) occlusion is associated with LD in patients with acute ischaemic stroke.18–20 Given the ligation of feeding arteries was needed in bAVM surgery,21 we inferred that resection of AF-bAVMs with AChA supplication might be at the risk of postoperative LD. Therefore, in the present study, we considered the association between AChA feeding and postoperative LD. Additionally, with a higher proportion of AChA supply, nidus involving segment IV was divided into type IVa (nidus with AChA feeding) and type IVb (nidus without AChA feeding).
Surgery
All patients were formally discussed in a multidisciplinary bAVM conference and a consensus treatment decision was made. Detailed information on surgery candidates selection and surgical resection was described in online supplemental material 1.
Language function evaluation and follow-up
Language function was assessed by an experienced neurosurgeon (HL) with the Chinese version of Western Aphasia Battery at 7 days (short-term outcomes) and at the last follow-up (long-term outcomes) after surgery. Detailed information on language function evaluation was provided in online supplemental material 1.
Study variables and definition
Patient demographics, including age, sex and LD history, were collected. Angioarchitectural variables for each AF-bAVM, such as size, diffuseness, deep draining veins, haemorrhagic presentation, deep perforating artery supply and Spetzler-Martin grading, were determined from preoperative neuroradiological data. Three deep perforating arteries were evaluated, including the AChA, posterior choroidal arteries and lateral lenticulostriate arteries (LLSAs). Diffuseness was determined from preoperative angiograms and TOF images. Haemorrhagic presentation was defined based on the combination of medical history and radiological findings.
Statistical analysis
Variables were compared between patients with and without postoperative LD. Continuous variables are summarised as the means±SD and categorical variables are summarised as frequency counts and percentages. Wilcoxon rank-sum tests, t-tests and χ2 tests were used as appropriate. Univariate and multivariate logistic regression analyses were used to assess the risk factors for short-term and long-term postoperative LD. Variables with p<0.05 in the univariate analysis were then used in the multivariate analysis. Statistical tests were considered significant at p<0.05. ORs are presented with 95% CIs. Statistical analyses were performed with the statistical software package SPSS (V.22.0.0).
Based on the results of multivariable logistic regression analysis, a grading system was developed for the prediction of long-term LD. We organised each risk factor into meaningful categories and calculated the risk score for each category based on the regression coefficient. Then, the predictive accuracy was assessed using the area under the receiver operating curves (AUCs). Because we used all the data to build our prediction models, which could result in overly optimistic predictions, we performed a fivefold cross-validation. The diagnostic utility of the model was analysed in R (V.4.0.2).
Results
Clinical characteristics
Between January 2013 and May 2020, 135 patients with AF-bAVMs were included in this study (table 1). There were 90 (66.7%) male patients and 45 (33.3%) female patients, with a mean age of 28.1±12.3 years. Sixty-nine (51.1%) patients had preoperative haemorrhage. The mean nidus diameter was 35.9±14.4 mm. Twenty-eight patients (20.7%) had a diffuse nidus, and 107 patients (79.3%) had a compact nidus. The number of patients with niduses involving segments I, II, III, and IV of AF was 51 (37.8%), 19 (14.1%), 61 (45.2%), and 25 (18.5%), respectively. The mean follow-up was 50.2±24.9 months.
Baseline characteristics of patients with AF-bAVMs
Clinical outcomes
All of the patients achieved complete resection of the bAVMs confirmed by the postoperative digital subtraction angiography (DSA) and none of the patients received postoperative stereotactic radiosurgery (SRS) or embolisation. Sixty-two (45.9%) patients suffered from postoperative short-term LD. At a mean follow-up of 50.2±24.9 months after surgery, long-term LD was found in 14 (10.4%) patients. Significant differences in nidus size (p=0.011), AChA feeding (p=0.013), nidus diffuseness (p=0.029), LD history (p=0.009) and segment II involvement (p=0.009) were found between patients with and without short-term postoperative LD. For long-term surgical outcomes, preoperative haemorrhage (p=0.006), AChA feeding (p=0.002), LLSA feeding (p=0.017), LD history (p<0.001) and segment II involvement (p=0.001) were significantly associated with an increased risk of LD. No significant difference was found in other factors (online supplemental table 1).
Risk factors for LDs
According to univariable logistic regression analyses, nidus size (p=0.013), AChA feeding (p=0.025), nidus diffuseness (p=0.032), LD history (p=0.013) and segment II involvement (p=0.013) were considered significant predictors of short-term LD. Multivariable logistic regression analyses showed that nidus size (p=0.007), LD history (p=0.009) and segment II involvement (p=0.030) were independent risk factors for short-term LD (table 2). Meanwhile, univariable logistic regression analyses identified patient age (p=0.028), preoperative haemorrhage (p=0.015), AChA feeding (p=0.001), LLSA feeding (p=0.011), LD history (p<0.001) and segment II involvement (p<0.001) as significant predictors of long-term LD. Multivariable logistic regression analyses showed that patient age (p=0.023), AChA feeding (p=0.001), LD history (p=0.001) and segment II involvement (p=0.002) were significantly associated with long-term LD (table 3).
Univariable and multivariable analyses of risk factors associated with short-term LD
Univariable and multivariable analyses of risk factors associated with long-term LD
Surgical outcomes of bAVMs involving different AF segments
Segment II involvement was an independent risk factor for short-term and long-term LD. Of the 19 patients with bAVMs involving segment II, 14 (73.7%) had short-term LD, and 7 (36.8%) had long-term LD after surgery. However, involvement of segments I, III or IV was not statistically related to postsurgical LD. Additionally, the language function recovery time differed in the nidus involving different segments of AF (figure 2). The mean recovery time was 4.15±2.44, 9.71±6.95, 5.21±4.69, and 5.00±4.30 months in segmentation I, II, III, and IV involvement, respectively. Compared with the involvement of segments I and III, the language function of patients with bAVMs located in segment II required a significantly longer time to recover (segment I involvement vs II, p=0.024; segment III involvement vs II, p=0.044). Moreover, we divided segment IV into IVa and IVb according to the existence of AChA feeding. Patients with bAVMs involving IVa showed a significantly higher incidence of short-term postsurgical LD than those with IVb involvement (p=0.001). Meanwhile, the association between long-term LD and segment IVa involvement showed a trend toward significance (p=0.081) (online supplemental table 2).
Violin graph showing the difference in postoperative LD recovery time according to the AF segment where the nidus was located. The language function of patients with bAVMs located in segment II required a significantly longer time to recover than those with the involvement of segments I and III (segment I involvement vs II, p=0.024; segment III involvement vs II, p=0.044) (*p<0.05 based on Wilcoxon rank-sum tests). AF, arcuate fasciculus; bAVMs, brain arteriovenous malformations; LD, language deficit.
Grading system for long-term LD
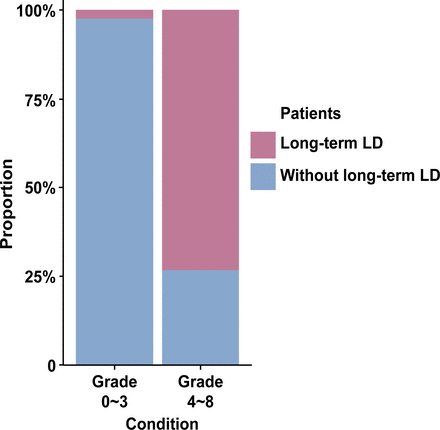
We developed a grading system to predict long-term postsurgical LD. Four variables (patient age, AChA feeding, LD history and segment II involvement) that maintained their prognostic significance after the multivariate analysis of long-term postoperative LD were used to construct a grading system (table 4). A score was calculated for each patient by adding the points corresponding to the patient’s risk factors, which ranged from 0 to 8. According to the receiver operating curve analysis that tested the predictive value of the grading system for long-term LD in all patient cohorts, the AUC was 0.921 (95% CI 0.836 to 1.000), and the cut-off point for long-term LD was 4 (figure 3). Based on the cut-off point, patients were divided into two groups: low-grade (grade 0~3) and high-grade (grade 4~8) groups. In the high-grade group, 11 of 15 (73.3%) patients suffered from long-term postoperative LD; however, only 3 of 120 (2.5%) patients had long-term LD in the low-grade group (figure 4).
ROC analyses of the grading system for predicting long-term postoperative LD in all patient cohorts. The AUC was 0.921 (95% CI 0.836 to 1.000), and the cut-off point was 4. AUC, area under the receiver operating curve; LD, language deficit; ROC, receiver operating curve.
Stacked bar chart showing the proportions of patients with or without long-term postoperative LD in low-grade and high-grade groups. In the high-grade group, 73.3% of patients suffered from long-term postoperative LD; however, only 2.5% of patients had long-term LD in the low-grade group. LD, language deficit.
Predictive grading system for long-term postoperative LD risk
To evaluate whether the predictive accuracy of our grading system was overly optimistic, we performed fivefold cross-validation of the data. The fivefold cross-validation resulted in estimates similar to the long-term LD grading system with an AUC of 0.926 compared with 0.921, suggesting that the model was not overly optimistic.
Discussion
At present, the long-term language outcome of AF-bAVMs remains poorly characterised. In addition, a grading system for the presurgical evaluation of long-term postoperative LD risk is needed. The use of proper patient selection criteria may improve the safety of AF-bAVM resection. In the current study, we demonstrated the risk factors associated with postoperative LD, especially with long-term LD, in a cohort of 135 patients with a mean follow-up of 50.2 months after surgery. Additionally, we proposed a new method to segment the AF and confirmed our hypothesis that different AF section involvement does have an influence on surgical outcome. Finally, based on the results of multivariable logistic regression analysis of long-term LD, we developed a grading system with satisfactory predictive performance.
Long-term postoperative LDs
According to present data, 62 (45.9%) patients had surgery-related LD at 1 week after surgery, which is similar to our previous study (46.4%). However, the incidence of postoperative long-term LD (10.4%) was nearly half of our previous literature (20.3%).2 We attributed this to the longer follow-up time of the current study, which could help us distinguish a more precise cohort of patients with postoperative LD hardly to recover. Our previous study identified preoperative LD history, nidus size and long segment of AF involvement were risk factors for long-term postoperative LD.2 In this study, besides the LD history, we identified that the patient age and AChA feeding should also be considered during candidate selection, with the exception of nidus size. Additionally, based on a new method of AF segmentation, we identified that the involvement of the trunk of the AF between Broca’s area and the inferior parietal lobule was also a risk factor for long-term postoperative LD.
Surgical outcomes of different AF segments
Based on the characteristics of the AF course and clinical practice, we divided the AF into four segments. According to the present data, the involvement of segment II was significantly associated with short-term and long-term postoperative LD and a longer recovery time from LD following surgery. This was consistent with our clinical practice. We speculated that this was because of the characteristics of the course of AF. The fibres in segments I, III and IV were scattered, whereas the fibres were gathered into a trunk in segment II.1 14–16 Compared with the other three segments, the fibres in segment II were more concentrated, which means that more fibres may be exposed to injury during surgery. This may explain the strong correlation between poor outcomes and involvement of segment II.
Effect of AChA feeding
In this study, we found that a nidus supplied by the AChA was significantly related to long-term postoperative LD and had a trend toward a significant relationship with short-term LD. Moreover, between the two subtypes of segment IV divided by the AChA feeding condition, a significant difference in short-term LD and a trend-level difference in long-term LD were observed. During the AF-bAVM surgery, the feeding arteries must be ligated to facilitate resection.21 It has been demonstrated that in patients with acute ischaemic stroke, occlusion of AChA in the dominant hemisphere could result in LD, and disconnection between the thalamus and the cortex may account for its occurrence.18–20 Hence, there may exist a danger of LD following the resection of AF-bAVMs with AChA feeding. However, the significance of AChA feeding in patients with AF-bAVMs is undetermined. Our study demonstrated the adverse effects of AChA feeding on long-term postoperative language function, and we added it to the grading system.
Lesion size and postoperative LD
In the present study, nidus size was considered as an independent predictor for short-term postoperative LD, which was consistent with our previous study.2 For patients with larger bAVMs, there are often problems such as longer operation time, larger amounts of blood loss and a greater amount of normal adjacent neural tissue exposed to injury. However, different from our previous literature, the nidus size was not associated with long-term postoperative LD. We supposed this was attributed to the ability of interhemispheric reorganisation of language function.22 Given the recruitment of the right hemisphere, the impact of nidus size on long-term postoperative LD might gradually fade over time.
Other factors
This study also found that a preoperative LD history was associated with short-term and long-term LD following AF-bAVM resection. Patients with preoperative LD may suggest that the nidus is close to the eloquent area that is crucial to language function, and the operation may aggravate the damage to the eloquent areas. Additionally, we identified that patient age was related to long-term postsurgical LD. Patient age seems to have a notable influence on neural function plasticity after invasive treatment, possibly reflecting decreases in brain plasticity as patients grow older.23
Meanwhile, the present data indicated that the involvement of the Broca’s or Wernicke’s area was not associated with postoperative LD (online supplemental table 3), which is similar to our previous study.2 It could be explained that the patients enrolled in this study were all with bAVMs involving the structure crucial to language function, which relatively reduced the association between the Broca’s and the Wernicke’s area and postoperative language outcomes.
Grading system for long-term LD
Currently, there is no specific grading system to assess long-term LD following AF-bAVM resection. In this study, we developed a grading system based on AF segments. Moreover, the effect of AChA feeding was also taken into consideration. According to our data, the grading system for long-term LD had favourable predictive accuracy. Based on the present data, a low-grade AVM (grades 0~3) may strengthen the recommendation for surgery. Conversely, for patients with high-grade AF-bAVMs (grades 4~8), treatment-related risks must be balanced against the natural history of bAVMs.24 Additionally, cautious assessment and adequate communication with patients and their families are necessary to select treatment strategies. Multimodality treatments with microsurgery performed as a definitive treatment might be recommended for their potential in decreasing operative risks and protecting neurological functions.25 Further studies are needed to evaluate the clinical benefits of this treatment strategy.
The management of unruptured bAVMs remains controversial. A Randomized Trial of Unruptured Brain Arteriovenous Malformations (ARUBA) was the first trial aimed to compare the effects of medical and interventional therapy for unruptured bAVMs. Its results suggest that conservative medical management is superior to interventional therapy in treating unruptured bAVMs.26 ARUBA stood as the most influential multicentre, prospective, randomised trial of unruptured bAVMs, which guided the treatment of unruptured bAVMs. However, its findings were met with profound criticism, including the lack of standardisation of intervention, the inclusive definition of stroke, the selection bias and the short follow-up period.27–29 Several observational studies subsequently published their results of ARUBA-eligible patients with invasive treatment. These studies indicated that over a reasonable follow-up period exceeding the latency period, interventional therapy for unruptured bAVMs might lead to better outcomes than natural history.30–32 Regardless of the dispute, the consensus is that appropriate selection for patients and treatment modalities is the most crucial. In the present study, to safely and efficiently treat AF-bAVMs, we provided the grading system for selecting surgical candidates in patients with AF-bAVMs.
Limitations
This study has some limitations. First, this study was conducted in a single centre. Second, the retrospective nature may make it difficult to avoid selection bias, information bias and confounding factors. Third, since the postoperative MRI is not routinely acquired in our study, further study with careful design is needed to identify the association between the postoperative LD and the areas of infarct or oedema following bAVM resection. Finally, although to our best knowledge, this study is the largest study termed patients with AF-bAVMs, and our internal validation with a cross-validation method showed favourable diagnostic performance, further external validation studies are needed to generalise our grading system.
Conclusions
The involvement of the trunk of the AF between Broca’s area and the inferior parietal lobule, a nidus supplied by the AChA, older patient age and a patient history of LD are associated with long-term postoperative LD. A grading system combining these factors demonstrated favourable predictive accuracy for the long-term language outcomes of AF-bAVMs.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Written informed consent was obtained from all participants or their legally authorised representative. The study was approved by the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University (KY2016-031-01).
Acknowledgments
We are grateful to Yi Zhai (China National Clinical Research Center for Neurological Diseases) for her advice in statistical assistance.
Footnotes
YJ and SZ contributed equally.
Contributors YJ designed the study, analysed and interpreted the data and drafted the manuscript for intellectual content. SZ designed the study, collected and analysed the data and drafted the manuscript for intellectual content. HL and JW collected and interpreted the data and revised the manuscript for intellectual content. JW, RH and JW collected the data and revised the manuscript for intellectual content. SW and JZZ designed the study and revised the manuscript for intellectual content. YC provided overall oversight of the research and acted as the guarantor who accepted full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish. YJ and SZ contributed equally to this work.
Funding This study was supported by the National Natural Science Foundation of China (Grant No. 81901175, Principle Investigator: Yuming Jiao), the 'National key research and development program of China during the 13th Five-Year Plan Period' (Grant No. 2016YFC1301803, Principle Investigator: Professor Yong Cao).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.