Abstract
Background Healthy plasma therapy reverses cognitive deficits and promotes neuroplasticity in ageing brain disease. However, whether healthy plasma therapy improve blood–brain barrier integrity after stroke remains unknown.
Methods Here, we intravenously injected healthy female mouse plasma into adult female ischaemic stroke C57BL/6 mouse induced by 90 min transient middle cerebral artery occlusion for eight consecutive days. Infarct volume, brain atrophy and neurobehavioural tests were examined to assess the outcomes of plasma treatment. Cell apoptosis, blood–brain barrier integrity and fibroblast growth factor 21 knockout mice were used to explore the underlying mechanism.
Results Plasma injection improved neurobehavioural recovery and decreased infarct volume, brain oedema and atrophy after stroke. Immunostaining showed that the number of transferase dUTP nick end labelling+/NeuN+ cells decreased in the plasma-injected group. Meanwhile, plasma injection reduced ZO-1, occluding and claudin-5 tight junction gap formation and IgG extravasation at 3 days after ischaemic stroke. Western blot results showed that the FGF21 expression increased in the plasma-injected mice. However, using FGF21 knockout mouse plasma injecting to the ischaemic wild-type mice diminished the neuroprotective effects.
Conclusions Our study demonstrated that healthy adult plasma treatment protected the structural and functional integrity of blood–brain barrier, reduced neuronal apoptosis and improved functional recovery via FGF21, opening a new avenue for ischaemic stroke therapy.
Introduction
Ischaemic stroke is one of the most critical cause of long-term disability and death in the world.1 Due to the rapid development of irreversible brain injury and lack of effective therapy, ischaemic stroke often leaves patients with long-term disability.2 Therefore, investigating novel therapeutic approaches is vital for ischaemic stroke treatment.
Blood–brain barrier (BBB) plays a crucial role in maintaining brain homoeostasis by isolating the central nervous system (CNS) from the peripheral circulation and strictly controlling the molecular exchange between the brain and peripheral blood circulation.3 4 BBB breakdown is a hallmark of ischaemic stroke.5 The structural and functional disruption of BBB allows circulating antigen, leukocytes and inflammatory cytokines to flux into the brain parenchyma, which induces neuroinflammation, brain oedema and eventually neuronal cell death.6 7 Thus, preserving the integrity and function of BBB after ischaemic stroke would be essential for improving the ischaemic stroke outcomes.
Emerging evidence suggested that systemic circulation factors, such as insulin-like growth factor, tissue inhibitor of metalloproteinases 2, growth hormone-releasing hormone, gonadotropin-releasing hormone, growth and differentiation factor 11 and glycosylphosphatidylinositol -specific phospholipase D1, affect CNS function in normal ageing and diseases.8–10 Bloodborne factors from young donors could significantly improve stem cell function and ameliorate cardiac hypertrophy in aged mice.8 11–13 Young blood factors rejuvenated synaptic plasticity and enhanced cognitive function of aged mice,14 while old circulating factors inhibited myogenesis and neurogenesis in young mice.15 Studies revealed that human cord plasma contained plasticity-enhancing proteins, which target ageing-associated or disease-associated hippocampal dysfunction.10 Another study showed that bloodborne fibroblast growth factor 21 (FGF21) promoted remyelination after CNS injury.16 Health young mouse blood replacement after ischaemic brain injury reduced ischaemic brain injury by attenuating inflammatory cascade.17 A recent study indicated that circulatory proteins could permeate the healthy adult mouse brain.6 Together, we hypothesise that healthy young plasma could be a promising therapeutic approach for treating ischaemic stroke .
In this study, we explore the effect of young healthy plasma on BBB integrity and function in mice after transient middle cerebral artery occlusion (tMCAO). We also investigate the underlying mechanism of plasma therapy.
Materials and methods
Experimental design
Adult female C57BL/6 mice were obtained from Jiesijie Laboratory Animal Co, Shanghai, China. FGF21-KO mice (C57BL/6 background) purchased from Cyagen Biosciences Inc. Suzhou, China. Animals were reared in a 12-hour light-dark cycled constant temperature room at 24℃±1℃ with free access to water and food.
Mouse plasma collection
Plasma was collected as described.10 Briefly, the 2-month-old healthy mice were anaesthetised with 5% (W/V) chloral hydrate, 700–900 µL whole blood was collected via posterior venous plexus in an ethylenediaminetetraacetate (EDTA) coated centrifuge tube, centrifuged at 3500 rpm for 15 min at 4℃. The supernatant was aspirated and frozen at −80℃ until use. Before injection, the plasma was combined and dialysed against cold PBS to remove EDTA. FGF21 concentration of blood plasma was evaluated by using ELISA kit (MF2100, R&D System, MN) following manufacture’s instruction.
tMCAO in mice
tMCAO was performed as previously described.18 19 Briefly, mice were anaesthetised with 2% isoflurane. The body temperature was kept at 37°C±0.5℃ with a heating mat (RWD Life Science, Shenzhen, China). The middle cerebral artery was occluded with a silicon-coated 6–0 suture which was inserted from the external carotid artery to the internal carotid artery until it reached the middle cerebral artery. Blood flow was evaluated using laser Doppler flowmetry (LDF). The cortical blood flow descended below 20% of the baseline was regarded as a success of occlusion. The suture was removed 90 min after the occlusion, reperfusion was confirmed by using LDF. One hour after reperfusion, mice were intravenously injected 100 µL plasma or saline once a day for eight consecutive days.
Neurological behavioural evaluation
Behavioural tests including Modified Neurological Severity Scores (mNSS), rotarod test, elevated body swing test (EBST) and corner test were performed by a researcher blind to the treatment assignment before surgery and at 1, 3, 7 and 14 days after tMCAO. The mNSS was used to evaluate the motor, sensory, balance and reflex function. The score ranges from 0 to 14; the higher the number, the more severe the neurological deficits.20
The rotarod test was conducted to further evaluate motor function of stroke mice. Briefly, all animals were trained on the gradually speeding rotarod (40 revolutions per min for 2 min) for three consecutive days before tMCAO. At 0–14 days after tMCAO, rotarod test was performed to determine the motor function of tMCAO mice.21 22 The duration that the mice stayed on the rotating rod was recorded and analysed by averaging three trials.
For EBST, mice were suspended by the tail and held vertically at about 10 cm above a surface, the direction of first body swing of the upper body at >10° angle to either side of the vertical axis was recorded. The mouse was placed back into the cage to reposition before the next swing. Twenty trials were repeated over a 5–10 min period.
For corner test, mice were put facing the corner of 30° angled boards. The direction of the mouse turned was recorded. For each mouse, repeat the procedure ten times with 5 min interval between each test.
Brain infarct volume and atrophy assessment
Twelve serials of frozen sections at 20 µm in thickness with 200 µm intervals were collected and stained with 0.1% cresyl violet (Meilun, Dalian, China). The infarct area in each brain section was evaluated by using the Image J software (National Institute of Health, Bethesda, Maryland USA) as previously described.19 The infarction volume of the brain was determined by applying the following equation, where h indicates the intervals between each section and S stands for the infarction area (mm2) in each section.

Brain oedema and brain atrophy were calculated with the following formula: [(Ipsilateral hemisphere volume-Contralateral hemisphere volume)/ Contralateral volume]×100%.23
Immunostaining and quantification
The immunostaining was conducted as previously described.19 Four brain sections with 200 µm intervals were fixed in cold methanol or 4% paraformaldehyde for 10 min at 4°C and then incubated in phosphate buffer saline (PBS) containing 0.3% Triton X-100 for 10 min, followed by blocking with 10% bovine serum albumin for 1 hour at room temperature. The sections were then incubated with antibodies against occludin (1:100, Invitrogen), zonula occludens-1 (ZO-1, 1:100, Invitrogen), claudin-5 (1:100, Invitrogen) and cluster of differentiation 31 (CD-31,1:200, R&D system) for at 4°C 14 hours. In Situ Cell Death Detection Kit (Roche, Diagnostics, Basel, Switzerland) was used for transferase dUTP nick end labelling (TUNEL) staining. The brain sections were reacted with NeuN antibody at 4°C for 14 hours, and then incubated with TUNEL for 1 hour at 37°C.
IgG extravasation was evaluated as previously described.20 24 Briefly, brain cryosections were stained with avidin biotinylated enzyme complex reagent (Vector Labs, Burlingame, California, USA). 3,3’-diaminobenzidine (DAB) staining was used for the visualisation of immune reactivity.
Immunostaining results was quantified as in our previous studies described.19 23 Four brain sections with 200 µm interludes were stained for each mouse, four fields were randomly chosen from each brain-section for confocal microscopy imaging (Leica, Solms, Germany). The tight junction gap length was displayed as a percentage (%) of the gap length to the complete blood vassal length.20 The IgG leakage was quantified with Image J Software immunohistochemistry image analysis toolbox.
Western blot analysis
Western blot was conducted as in our former studies reported.19 25 Briefly, the brain was harvested at 3 days after tMCAO and peri-infarct brain tissues were collected from the ipsilateral hemisphere. A 40 µg total protein from each brain sample was loaded and electrophoresis on a 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) gel, then transferred to the polyvinylidene fluoride (PVDF) transfer membrane, followed by reacting with primary antibody FGF21 (1:1000, Abcam) or β-actin (1:1000, Invitrogen) at 4℃ overnight. Subsequently, the membrane was incubated with horseradish peroxidase (HRP) tagged secondary antibodies and reacted with enhanced chemiluminescence substrate. Western blot image was collected using an imaging system (Bio-Rad, Hercules, California, USA). The relative levels of FGF21 were normalised to that of β-actin.
Real-time PCR analysis
Total RNA was extracted by TRIzol Reagent (Invitrogen) following the manufacturer’s instruction. The RNA was evaluated by spectrophotometer (NanoDrop1000, Thermo, Wilmington, Delaware, USA). cDNA was synthesised using the Reverse Transcriptase Kit (Yeasen, Shanghai, China). The PCR reaction condition is as previously reported: 95℃ for 30 s followed by 40 cycles of 95℃ for 5 s and 60℃ for 30 s.19 The primer sequences are provided in table 1. The relative mRNA expression level was determined by the Δ/Δ Ct method.25
Real-time PCR primers
Statistical analysis
All data were presented as mean±SD The SPSS V.18.0 software (SPSS) and GraphPad PRISM V.6.0 software were employed for statistical analysis and comparisons of data. The G* Power was used to estimate the minimal sample size and power analysis. Multiple comparisons were evaluated by one-way analysis of variance post Bonferroni or the Tamhane test (heterogeneity of variance). A p<0.05 was regarded statistically significant.
Results
Healthy blood plasma injection promoted neurobehavioural recovery and reduced brain infarct volume in tMCAO mice
To test whether healthy plasma attenuated ischaemic brain injury and facilitated the neurobehavioural recovery, neurological outcomes of tMCAO mice were evaluated after plasma treatment. Our results showed that the plasma-injected group exhibited a lower mNSS score and longer time on the rotarod than the saline-injected group at day 7 and 14 of tMCAO (figure 1A,B; p<0.01). The body swing test (EBST) and corner test also demonstrated that the plasma-injected group showed improved neurobehavioural outcomes compared with the control (figure 1C,D; p<0.05), indicating that plasma injection could ameliorate neurological deficiency, improved motor function in tMCAO mice. Furthermore, the Cresyl violet staining results (figure 1E,H) showed that healthy plasma injection attenuated brain infarct volume (14.24±2.89 mm3) and atrophy (5.54±0.82 mm3) compared with the control (29.81±3.06 mm3, 13.86±1.67 mm3) at 3 days and 14 days after tMCAO (figure 1G,I,J; p<0.05). Brain oedema volume was also reduced in the plasma-injected group (20.54%±2.45%) compared with the control group (48.14%±4.07%) after 3 days of tMCAO (figure 1F, p<0.05).
Healthy plasma treatment promoted neurobehavioural recovery, reduced infarct volume and atrophy volume in tMCAO mice bar graphs showed that the results of modified neurological severity score (A), rotarod test (B), EBST (C) and corner test (D) in plasma-injected and control mice at 1, 3, 7 and 14 days after tMCAO. Representative images of cresyl violet-stained brain sections of plasma-injected and control mice at 3 days (E) and 14 days (H) after tMCAO. Bar graph showed quantification of brain oedema and infarct volume in plasma-injected and control mice at 3 days (F, G) and brain atrophy volume at 14 days (I, J) after tMCAO. Data are mean±SD, n=12 per group. *p<0.05, **p<0.01. ***p<0.001, plasma-injected versus control mice. EBST, elevated body swing test; tMACO, transient middle cerebral artery occlusion.
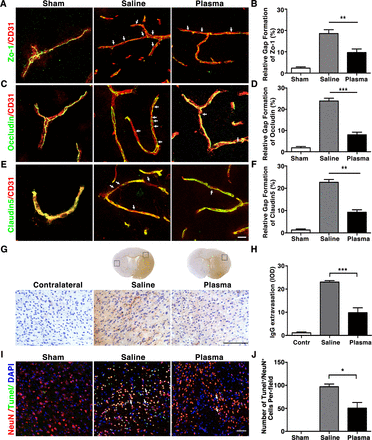
Healthy blood plasma injection attenuated BBB disruption and neuronal apoptosis
To test the effects of healthy plasma in preserving BBB integrity of tMCAO mice, we performed coimmunoassaying of BBB related tight junction proteins ZO-1, occludin and claudin-5 with endothelial cell marker CD31 at 3 days after tMCAO. Results showed that ZO-1, occludin, claudin-5 were continuously expressed on the endothelial cell edge of cerebral microvessels in the sham mice (figure 2A,C,E). However, ischaemic injury disrupted this continuity. It was noted that healthy plasma injection attenuated ZO-1 (9.67%±0.82%), occludin (7.99%±0.61%) and claudin-5 (9.27%±1.10%) gap formation and discontinuity compared with control (18.55%±1.05%, 23.88%±0.67% and 22.68%±1.26%) (figure 2B,D,F; p<0.01). IgG immunostaining result showed that IgG extravasation was reduced in the plasma-injected group (integrated optical density (IOD)=9.81±2.11) compared with the controls (IOD=23.04±0.63) after tMCAO (figure 2G,H; p<0.001). We further evaluated the effects of healthy plasma in neuronal apoptosis after tMCAO by performing neural nucleus marker NeuN and TUNEL staining. We found that plasma injection decreased the amount of NeuN+/TUNEL+ apoptotic neurons (50±12 cells) than the control group (97±5 cells) at 3 days after tMCAO (figure 2I,J; p<0.05).
Healthy plasma reduced BBB disruption and neuronal apoptosis in tMCAO mice photomicrographs showed that the endothelial cell marker CD31 (red) with BBB tight junction proteins (green), ZO-1 (A), occludin (C) and claudin-5 (E) in the peri-infarct area of the striatum in sham, salineinjected and plasma-injected mice. Arrows indicate that the gaps in BBB tight junctions. Scale bar=10 µm. Bar graphs showed that quantification of BBB tight junction proteins ZO-1 (B), occludin (D) and claudin-5 (E) in sham, saline-injected and plasma-injected mice at 3 days of tMCAO. Data are mean±SD, n=4 per group. **p<0.01. ***p<0.001, Plasma-injected versus control mice. (G) Photomicrographs showed that the IgG (brown) in the peri-infarct area of the striatum in sham, saline-injected and plasma-injected mice. Scale bar=75 μm. Bar graphs showed the quantification of IgG proteins (H) in sham, saline-injected and plasma-injected mice at 3 days of tMCAO. Data are mean±SD, n=5 per group. ***p<0.001, plasma injected versus control mice. (I) Photomicrographs showed that the NeuN (red) and TUNEL (green) doublepositive cells in the peri-focal area in the plasma-treated and control mice at 3 days of tMCAO. Arrows indicate NeuN+/TUNEL+ cells, scale bar=50 μm. (J) Bar graphs showed the quantification of TUNEL+ cells in plasma-treated sham, and control mice at 3 days of tMCAO. Data are mean±SD, n=5 per group. * p<0.05, plasma injected versus saline injected. BBB, blood–brain barrier; tMCAOM, transient middle cerebral artery occlusion; TUNEL, transferase dUTP nick end labelling; ZO-1, zonula occludens-1.
Blood plasma treatment inhibited neuroinflammation after tMCAO
As inflammatory cytokines contribute to the BBB disruption during brain ischaemia, we further investigated the level of inflammatory cytokines tumour necrosis factor-α (TNF-α), interleukin (IL-6), IL-1β, IL-18, interferon gamma (INF-γ), IL-17 and inducible nitric oxide synthase (iNOS) mRNA expression in the plasma-injected mice at 3 days after tMCAO. Results indicated that plasma injection significantly decreased TNF-α (1.08±0.27 fold), IL-6 (1.59±0.39 fold), IL-1β (0.86±0.22 fold), IL-18(0.58±0.10 fold), INF-γ (0.75±0.30 fold), IL-17 (1.18±0.34 fold) and iNOS (1.02±0.15 fold) the expression of these inflammatory cytokines compared with the controls (respectively, 3.46±0.58, 3.59±0.56, 1.98±0.30, 1.74±0.28, 5.61±1.87, 11.65±5.56 and 2.85±0.32 fold) (figure 3A–G; p<0.05).
Healthy plasma reduced inflammatory response in the tMCAO mice brain bar graph showed that RT-PCR quantification of inflammatory factors TNF-α (A), IL-6 (B), IL-1β (C), IL-18 (D), INF-γ (E), IL-17 (F) and iNOS (G) mRNA expression in sham, saline-injected and plasma-injected mouse brain at 3 days of tMCAO. data are mean±SD, n=6 per group. *p<0.05; **p<0.01, plasma injected versus control mice. INF-γ, interferon-γ; IL-6, interleukin 6; iNOS, inducible nitric oxide synthase; tMCAO, transient middle cerebral artery occlusion; TNF-α, tumour necrosis factor-α.
Healthy blood plasma injection increased FGF21 and its receptor β-klotho expression in tMCAO mice
Evidence suggested that FGF21 promotes remyelination in BBB disrupted brain,16 and protects BBB integrity in traumatic brain injury.26 We found that plasma injection upregulated FGF21 expression (3.82±0.48-fold) at 3 days after tMCAO compared with the controls (2.1±0.34-fold) (figure 4A,B; p<0.05). Plasma injection also upregulated the expression of FGF21 receptors FGF receptor (FGFR)1 (1.25±0.28-fold), FGFR2(1.47±0.10-fold), FGFR3(1.73±0.13-fold) and coreceptor β-klotho (2.17±1.05-fold) in the ischaemic brain at the 3 days after tMCAO compared with the controls (respectively, 0.33±0.080, 0.63±0.09, 0.76±0.07 and 0.63±0.11 fold change to sham group) (figure 4C–G; p<0.05). Since FGF21 correlated with blood glucose regulation,27 28 we investigated whether blood glucose level changed after plasma injection into tMCAO mice. The results showed that the blood glucose concentration was significantly decreased in the plasma-injected mice compared with the control mice at 120 min after injection, which could be correlated with the upregulated FGF21 expression after plasma injection (online supplemental figure 1), p<0.05).
Supplementary data
Healthy plasma increased FGF21 and its' receptors expression in the tMCAO mouse brain photomicrograph showed that Western blot images of FGF21 bands (A) and bar graph (B) showed semiquantification of FGF21 protein expression in sham, saline-injected and plasma-injected mouse brain at 3 days of tMCAO. Data are mean±SD, n=3 per group. *p<0.05, plasma-injected versus control mice. RT-PCR quantification of FGF21 (C), β-klotho (D), FGFR1 (E), FGFR2 (F) and FGFR3 (G) mRNA expression in the sham, saline-injected and plasma-injected mouse brain at 3 days of tMCAO. Data are mean±SD, n=4 per group. *p<0.05; ** p<0.01, plasma-injected versus control. FGF21, fibroblast growth factor 21; FGFR1, fibroblast growth factor receptor; tMCAO, transient middle cerebral artery occlusion.
FGF21 knockout mice derived plasma reversed the plasma effect of neurobehavioural outcomes in ischaemic mice
To investigate whether FGF21 has played a critical role in the improvement of functional recover of plasma-injected tMCAO mice, plasma from FGF21-/- mice was injected into wild-type tMCAO mice. The plasma concentration of FGF21 in wild-type and FGF21-/- transgenic mice were evaluated (online supplemental figure 2, p<0.001) before the injection. The results showed that while the plasma from healthy wild-type mice improved neurobehavioural recovery of tMCAO mice, the injection of plasma from FGF21-/- mice did not improve neurobehavioural recovery. We also tested the effect of healthy wild-type plasma in treating tMCAO FGF21-/- mice. Interestingly, FGF21-/- tMCAO mice showed worse neurobehavioural outcomes and larger infarct volume than wild-type tMCAO mice (online supplemental figure 3A-F), and FGF21-/- tMCAO mice that were treated with plasma from healthy wild-type mice showed no improvement in neurobehavioural outcomes (figure 5A–D; p<0.05), suggesting that both endogenous FGF21 and exogenous FGF21 are important for improving the outcome of stroke. Cresyl violet staining showed that healthy plasma injection alleviated brain oedema (18.89%±3.21%) and infarct volume (8.97±1.01 mm3) at 3 days following tMCAO (figure 5E and G–H; p<0.05), as well as brain atrophy volume (6.18±1.55 mm3) at 14 days following tMCAO (figure 5F–J; p<0.05). However, compared with the healthy plasma-injected mice, the wild-type mice treated with the plasma derived from FGF21-/- mice exhibited larger brain oedema (34.11%±5.11%) and infarct volume (20.16±2.01 mm3) at 3 days of tMCAO and atrophy volume (17.20±2.98 mm3) at 14 days following tMCAO (figure 5G,H; p<0.05). In addition, the FGF21-/- tMCAO mice treated with healthy wild-type mice derived plasma showed negligible effects in neurobehavioural outcomes, brain oedema (44.83%±5.40%), infarct volume (19.71±2.66 mm3) and atrophy volume (15.12±1.86 mm3), suggesting that intrinsic FGF21 in the plasma is also important for improving neurobehavioural function after tMCAO.
Depletion of FGF21 in plasma reversed the beneficial effect of healthy plasma in the tMCAO mice bar graphs showed that neurological severity score (A), rotarod test (B), EBST (C) and corner test (D) in wild-type mice that treated with plasma from wild-type mice (WT+WT); wild-type mice that treated with plasma from FGF21-/- mice (WT+ FGF21-/- plasma); and FGF21-/- mice that treated with wild-type plasma (FGF21-/-+ WT plasma) at 1, 3, 7 and 14 days after tMCAO. (E, F) Images of cresyl violet-stained brain sections of WT+ saline group, WT+WT group, WT+ FGF21-/- plasma group, and FGF21-/-+ WT plasma group at 3 and 14 days of tMCAO. Dashed line showed infarct area in the ipsilateral hemisphere of the brain. Semiquantification of brain oedema and infarct volume in WT+ saline group, WT+WT group, WT+ FGF21-/- plasma group, and FGF21-/-+ WT plasma group at 3 days of tMCAO (G, H). Bbar graph showed that the brain atrophy volume and the volume ratio of the ipsilateral hemisphere and contralateral hemisphere in the WT+ saline group, WT+WT group, WT+ FGF21-/- plasma group and FGF21-/-+ WT plasma group at 14 days in the tMCAO mouse brain (I, J). Data are mean±SD, n=10 per group. *p<0.05, **p<0.01, ***p<0.001, FGF21-treated versus control mice. EBST, elevated body swing test; FGF21, fibroblast growth factor 21; tMCAO, transient middle cerebral artery occlusion.
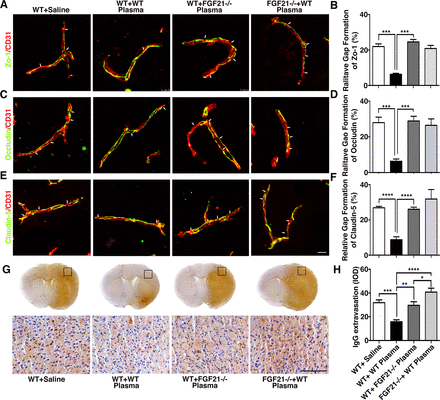
FGF21 knockout mice derived plasma injection increased BBB disruption after tMCAO
To explore if healthy plasma protected BBB integrity through FGF21, FGF21-/- mice derived plasma was injected into wild-type mice after tMCAO. We found that compared with mice treated with healthy wild-type plasma (respectively, 6.31%±0.56%, 6.37%±0.70%, 8.79%±0.97%), the mice treated with FGF21-/- plasma (24.58%±1.41%, 28.92%±1.49% and 26.09%±0.66%) or FGF21-/- tMCAO mice treated with healthy plasma (20.89%±1.73%, 26.50%±2.04%, and 31.87%±3.11%) showed increased ZO-1, occludin and claudin-5 gap formation (figure 6A–F, online supplemental figure 3). Furthermore, compared with wild-type tMCAO mice, FGF21-/- mice showed more gap formation (online supplemental figure 3G-I, p<0.001). Similarly, IgG immunostaining results also demonstrated that mice treated with FGF21-/- plasma or FGF21-/- mice treated with healthy plasma had higher IgG extravasation at 3 days after tMCAO (figure 6G–H; p<0.001).
Depletion of FGF21 in plasma diminished the beneficial effects of plasma-induced BBB protection in mice after 3 days of tMCAO coimmunostaining of endothelial cell marker CD31(red) with BBB (green) ZO-1(A), occludin (C) and claudin-5 (E) in the peri-infarct area of the striatum in wild-type mice that treated with saline (WT+ saline), wild-type mice that treated with wild mouse plasma (WT+WT), wild-type mice that treated with plasma from FGF21-/- mice (WT+ FGF21-/- plasma) and FGF21-/- KO mice that treated with wild-type plasma (FGF21-/- + WT plasma) at 3 days of tMCAO. Arrows indicate the gaps in BBB tight junction proteins, scale bar=10 μm. Quantification of gaps that formed on BBB tight junction proteins ZO-1(B), occludin (D) and claudin-5 (F) in the WT+ saline group, WT+WT group, WT+ FGF21-/- plasma group, and FGF21-/-+ WT plasma group at 3 days of tMCAO. Data are mean±SD, n=3 per group. **p<0.01. ***p<0.001. (G) Photomicrographs of IgG in the perifocal area of WT+ saline group, WT+WT group, WT+ FGF21-/- plasma group and FGF21-/-+ WT plasma group at 3 days after stroke. Scale bar=75 μm. (H) Bar graph showed semiquantification of IgG extravasation in the WT+ Saline group, WT+WT group, WT+FGF21-/- plasma group and FGF21-/-+WT plasma group. Data are presented as mean±SD, n=5 per group. * p<0.05; ****p<0.001, plasma versus control. BBB, blood-brain barrier; FGF21, fibroblast growth factor 21; tMCAO, transient middle cerebral artery occlusion; ZO-1, zonula occludens-1.
Discussion
Here, we demonstrated that injection of plasma into ischaemic mouse attenuated ischaemic infarct and atrophy volume, preserved BBB integrity, decreased neuroinflammation and neuronal apoptosis, and facilitated neurobehavioural recovery. We further demonstrated that FGF21 is a key protein involved in plasma mediated neuroprotective effects. Our data suggest healthy plasma transfusion could be a unique therapy for ischaemic stroke.
Recent studies suggested that blood contains factors that could combat ageing by rejuvenating stem cells in aged muscle,12 29 liver,12 heart,13 brain.9 11 15 In addition, plasma from young rodents ameliorated the prognoses and cognitive function in mice with Alzheimer’s disease.30 Administration of plasma from young rats into aged stroke rats decreased infarct volume and facilitated neurobehavioural recovery.31 On the contrary, administration of aged rats derived plasma into young stroke rats exacerbated brain injury and aggravated behaviour deficits, indicating that systemic factors could profoundly affect ageing-related impairments.31 Study found that substituting stroke mouse blood with healthy mice blood attenuated infarct volume and neurobehavioural deficits, and this therapeutic strategy significantly decreased neutrophils infiltration and diminished the MMP-9 expression, suggesting the potential therapy of plasma in ischaemic stroke.17 We confirmed the beneficial effects of plasma from healthy mice for treating ischaemic stroke mice. We demonstrated that injection of healthy mice derived plasma into ischaemic stroke mice reduced the infarct volume at 3 days after stroke and brain atrophy volume at 14 days after tMCAO. Importantly, plasma treatment also improved the neurobehavioural recovery of tMCAO mice. All these data suggested the potential use of plasma for ischaemic stroke therapy.
Our study demonstrated the multiple beneficial effects of healthy plasma in treating stroke mice. First, healthy plasma treatment significantly reduced BBB disruption after stroke. It is well known that stroke leads to BBB disruption and increased BBB permeability, resulted in invasion of inflammatory factors, neurotoxins and pathogens into the brain, and consequently, exacerbates functional deficits.5 We found that injection of healthy plasma into stroke mice decreased tight junction gap formation in microvessels, and reduced IgG extravasation. Second, healthy plasma treatment reduced inflammatory response. Increasing evidence from both clinical and experimental studies suggests that inflammation contributes to the secondary injury that occurs after stroke, mostly due to the infiltration of peripheral immune cells into the brain, and leads to the upregulation of inflammatory factors in the brain.32 Our results showed that healthy plasma treatment decreased neuroinflammation at 3 days after tMCAO, which was correlated with an attenuation of BBB disruption, as less inflammatory cells infiltrated into the brain. Third, healthy plasma treatment reduced neuronal apoptosis at 3 days after tMCAO. It is well documented that ischaemic brain injury leads to neuronal apoptosis, and its inhibition provides a critical therapeutic target for ischaemic stroke.4 33 We found that injection of healthy plasma reduced neuronal apoptosis by about 50%, which was attributed to the attenuated neuroinflammation in the ischaemic brain. Whether healthy plasma treatment could inhibit other neuronal death ways, such as necrosis, ferroptosis and pyroptosis needs further investigation.
FGF21, a growth factor, is predominantly secreted by the pancreas, regulates metabolic pathways.16 Recently, FGF21 has been shown to modulate systemic metabolism, inhibit inflammation and promote whiter matter recovery, supporting its therapeutic potential for neurological disorders.16 26 34 FGF21 protected the brain against BBB breakdown after ischaemic stroke and preserve tight junction integrity through FGFR1 upregulation and peroxisome proliferator-activated receptor γ pathway.26 35 In a toxin-induced demyelination study, FGF21 derived from the pancreas leak into the brain after injury and promoted myelination through interactions through β-klotho.16 Our study observed that infusion of healthy plasma into ischaemic stroke mice elevated FGF21 in the brain, as well as its receptors including FGFR1, FGFR2, FGFR3 and its coreceptor β-klotho, indicating that healthy plasma may exert its neuroprotective effects through FGF21. We noted that plasma injection decreased blood glucose in tMCAO mice. This phenomenon may be due to the regulatory effect of FGF21 in blood, as our study demonstrated upregulation of FGF21 level in mice that received blood from the donors. It is reported that FGF21 is a metabolic modulator and could decrease glucose level in diabetic or obese mice by increasing insulin sensitivity.28 35 36 We then found that infusion of plasma from FGF21-/- mice into wild-type tMCAO mice diminished the neuroprotective effect of plasma, which confirmed our hypothesis that FGF21 plays a key role in plasma induced neuroprotection and BBB protection. Interestingly, compared with wild-type tMCAO mice, FGF21KO mice showed worse neurobehavioural outcomes, larger infarct volume and more gap formation, and the injection of healthy plasma from wild-type mice into FGF21-/- tMCAO mice did not achieve beneficial effects for the outcome of stroke, indicating both endogenous FGF21 and exogenous FGF21 are both important for promoting the recovery of stroke mice. In addition, previous studies showed that FGF21 receptors and MAPK and Akt downstream effector molecules gene expression were changed in FGF21KO mice,28 37 which also decrease the protective effect of exogenous FGF21 on ischaemic stroke.
Sex differences in ischaemic stroke pathology are observed across experimental and clinical studies, which have profound implications for efficient stroke treatment.1 38 39 In our study, plasma was collected from female mice and injected into female mice that were subjected to ischaemic stroke. It is unclear whether plasma collected from different sexes contributed to different outcomes in ischaemic stroke. Age is another critical factor that influence ischaemic stroke prognosis. Studies showed that ageing significantly alter the cerebrovascular response to various stress and injuries.6 40 Although our study demonstrated that the young mice (2–3 months old) plasma transfusion protected against ischaemia-reperfusion injury of young stroke mice, whether plasma from aged mice still have neuroprotective effects in aged stroke mice needs to be clarified in future studies.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. The data that support the findings of this study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Animal surgery procedure and experimental protocol were approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University, Shanghai, China. All experiments were performed following the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health as well as the ARRIVE guidelines.
Footnotes
Contributors MM participated in the research design, all experimental procedures, animal surgery, data analysis and drafting of the first manuscript. LJ, YS and LD contributed to animal surgery, behavioural tests and data collection. HS, HZ, CL and YJ assisted with data collection. YT provided technical assistance for in vivo and in vitro experiments. ZZ and YW were involved in the discussion of the research design, the results and edited the manuscript. G-YY and YT supervised all aspects including research design, data analysis and manuscript preparation. All authors read and agreed to the final manuscript.
Funding This study was supported by grants from the Scientific Research and Innovation Program of Shanghai Education Commission 2019-01-07-00-02-E00064 (G-YY), National Key R&D, Program of China #2016YFC1300602 (G-YY), #2019YFA0112000 (YT), the National Natural Science Foundation of China (NSFC) projects 81771251 (G-YY), 81801170 (YT), 82071284 (YT), 81771244 (ZZ), 81974179 (ZZ), 81870921 (YW), and K. C. Wong Education Foundation (G-YY, no award/grant number).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.