Abstract
Background and purpose Current randomised controlled trials (RCTs) showed an uncertain benefit of haemostatic therapy on preventing haematoma expansion and improving the outcome in patients with intracerebral haemorrhage (ICH). This meta-analysis aims to systematically evaluate the effect of haemostatic agents on the prevention of haemorrhage growth in patients with high-risk spontaneous ICH predicted by CT signs in RCTs.
Methods A comprehensive search of PubMed, EMBASE and Cochrane library from 1 January 2005 to 30 June 2021 was conducted. RCTs that compared haemostatic agents with placebo for the treatment of spontaneous patients with ICH with high-risk haemorrhage growth were included. The primary endpoint was haematoma expansion at 24 hours. Other major endpoints of interest included 90-day functional outcome and mortality.
Results The meta-analysis included four RCTs that randomised 2666 patients with ICH with high-risk haemorrhage growth. Haemostatic therapy reduced the rate of haematoma expansion at a marginally statistically significant level when compared with placebo (OR 0.84; 95% CI 0.70 to 1.00; p=0.051). Subgroup analysis for patients with black hole sign on CT revealed a significant reduction of haematoma expansion with haemostatic therapy (OR 0.61; 95% CI 0.39 to 0.94; p=0.03). However, both the primary analysis and subgroup analyses showed that haemostatic therapy could not reduce the rate of poor functional outcome (modified Rankin Scale >3) or death.
Conclusions Haemostatic therapy showed a marginally significant benefit in reducing early haematoma expansion in patients with high-risk spontaneous ICH predicted by markers on CT scan. However, no significant improvement in functional outcome or reduction of mortality was observed.
INTRODUCTION
Spontaneous intracerebral haemorrhage (ICH) is one of the devastating strokes associated with the highest mortality and disability worldwide.1 Current surgical or medical treatment showed no clear benefit.2 3 Clinically, haematoma expansion is associated with early neurological deterioration and poor clinical outcome and a target of intervention.4 5 Haemostatic therapy has been shown to prevent haematoma expansion in patients with spontaneous ICH but with very limited evidence.6 In theory, haemostatic therapy is more suitable for patients with high-risk for ICH growth, such as patients with early CT signs of haematoma expansion.7 For this reason, high-risk haematoma growth patients with ICH with image markers, such as the spot sign,8 9 black hole sign10 and blend sign11 on CT scan were identified as candidates for haemostatic therapy studied in several randomised trials.6 12–14 With the recent completion of Tranexamic Acid for Acute ICH Growth prEdicted by Spot Sign (TRAIGE) trial12 and the results of other trials using haemostatic agents, a possible consistent trend for reducing haematoma expansion was seen. On the other hand, some of these trials showed conflicting results. We, therefore, performed a meta-analysis and systematic review of the available evidence to evaluate the effect of haemostatic agents on the prevention of haemorrhage growth in patients with high-risk spontaneous ICH predicted by positive signs on CT (spot sign, blend sign or black hole sign).
METHODS
Search strategy
The Preferred Reporting Items for Systematic reviews and Meta-Analyses statement for reporting systematic reviews and meta-analyses of randomised controlled trials (RCTs) were followed in this meta-analysis.15 PubMed, EMBASE and Cochrane library were searched for English peer-reviewed publications that were published from 1 January 2005 (year of publication of the Factor Seven for Acute Hemorrhagic Stroke phase Ⅱ trials (FAST Ⅱ) 16) to 30 June 2021. Randomised trials that compared haemostatic agents with placebo for the patients with ICH with high risk for haematoma expansion predicted by CT signs were identified. The following terms were used for the database search: ‘intracranial hemorrhages’, ‘hemorrhages’, ‘cerebral brain hemorrhage’, ‘tranexamic acid’, ‘t-AMCHA’, ‘AMCA’, ‘anvitoff’, ‘cyklokapron’, ‘spotof’, ‘transamin’, ‘mchafibrin’, ‘exacyl’, ‘Recombinant activated coagulation factor VII’, ‘rFVIIa’, ‘factor vii’, ‘factor seven’, ‘coagulation factor’, ‘aminocaproic acid’, ‘6-Aminohexanoic Acid’, ‘epsilon-Aminocaproic Acid’, ‘6-Aminocaproic Acid’, ‘CT’, ‘computed tomography’, ‘spot’, ‘black hole’, ‘blend’. Related reviews, clinical trial databases and the reference lists of all retrieved articles were also searched manually to identify relevant studies.
Selection criteria
According to the objective of this analysis, only randomised trials that reported original data on haematoma expansion incidence after any haemostatic agents use in high-risk patients with ICH predicted by positive signs on CT were considered for inclusion in the meta-analysis, and the needed data could be from overall analysis, subgroup analysis or post hoc analyses. All non-RCTs, including observational studies, reviews, editorials, letters and case reports, were excluded. The study subjects were restricted to adult patients with spontaneous ICH. In addition, haematoma expansion should be defined as the presence of any ICH growth at 24 hours (>33% or >6 mL from baseline volume).
Primary and secondary outcomes
The primary outcome was early haematoma expansion, defined as the presence of ICH growth measured at 24 hours (>33% or >6 mL from baseline volume). The secondary outcomes were poor functional outcome defined by a modified Rankin Scale (mRS) between 4 and 6 at 90±7 days and death at 90 days.
Data extraction and quality assessment
Two physicians independently extracted data from identified publications based on the inclusion criteria. Disagreements were resolved through the discussion among all authors until a consensus was reached. Methods specified in the Cochrane Handbook of Systematic Reviews were adopted in this study for the objective assessment of the included trials.17 Data on the total number of patients treated, duration of follow-up and specifics of the intervention and control groups were extracted from publications. The occurrence of the following events was extracted for individual trials and analysed separately for the haemostatic therapy group and the control group: number of patients with haematoma expansion, number of patients with an mRS of 4–6 (indicating reasonably poor functional outcome at 90 days) and number of deceased patients at 90 days. If the above data were not available, the unadjusted ORs, as the indicators of the efficacy, were extracted, as an alternative. Characteristic data were also retrieved. To assess the reliability of the pooled results, risks of bias for each article and the overall study were assessed and reported according to the recommendations of the Cochrane Handbook for Systematic Reviews of Intervention using Cochrane collaboration’s tool.17
Data synthesis and analysis
To combine the data from each study, the common-effect model with an inverse-variance (CE IV) method was used to calculate a summary estimate across all included studies. The OR estimates and associated 95% CIs for each of the endpoints were calculated. If only the subgroup of the trial met the inclusion criteria, analysis with and without the overall population from that trial would be carried out separately, as a part of sensitivity analysis. Subgroup analysis was prespecified based on the CT signs, types of haemostatic agents, following the methods outlined by CE IV for each outcome. Between-study and between-subgroup heterogeneities were evaluated by calculating the I2 statistic and the Cochrane Q (χ2) statistic, with a p value of 0.10 set for significance of the test of heterogeneity. The results of sensitivity analysis were showed graphically, demonstrating the influence of each study on the overall meta-analysis summary estimate. Funnel plots graphically showing the logarithm of the SE and the effect size to evaluate publication bias was also created. All tests were two-tailed with a p value of 0.05 considered significant. All analyses were performed using the Review Manager V.5.4 (The Nordic Cochrane Center, The Cochrane Collaboration, 2020, Copenhagen, Denmark) and Stata V.16.0 (StataCorp LLC, College Station, 2019, Texas, USA) software.
RESULTS
Description of study characteristics
The database search identified 2406 publications with one additional report from other sources (online supplemental 1). A total of four randomised trials with 2666 patients were eligible for the meta-analysis.6 12–14 Of the four trials, one enrolled both supratentorial and infratentorial patients with ICH6 and three enrolled only supratentorial patients with ICH .12–14 Two trials included patients with a positive spot sign only13 14 and one trial selected patients with at least one of the three positive CT signs (positive spot sign, black hole sign or blend sign).12 In another trial, CT signs were not considered as inclusion criteria,6 for which, the original data needed for the current analysis were extracted from the subgroup analysis and post hoc analysis.18 Tranexamic acid was used in three trials6 12 14 and recombinant activated coagulation factor VII (rFVIIa) in one trial.13 The control group in all trials received saline as the placebo. The average duration of follow-up was 90 days. The mean duration from the onset to treatment was 150 to 290 mins and the ICH volume at baseline was 14.6~24.0 mL. The risk of bias assessment for randomised clinical trials is presented in online supplemental 2 and all studies included in this review presented a low risk of bias and high quality. All trials adopted the randomised, double-blind, placebo-controlled design and intention-to-treat analysis for the primary analysis. More details of the design and characteristics of the included trials are provided in table 1 and online supplemental file 3.
Supplementary data
Supplementary data
Supplementary data
Characteristics of the studies included in the systematic review and meta-analysis
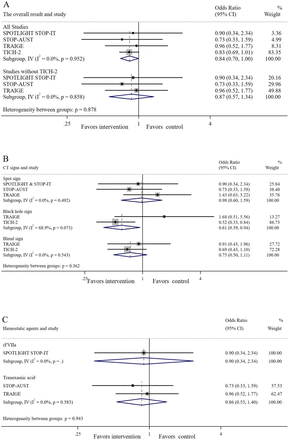
Haemostatic therapy reduced the rate of haematoma expansion at a marginally significant level, compared with placebo (27.4 vs 40.0% in the control group; OR 0.84; 95% CI 0.70 to 1.00; p=0.051), and the result had similar trends but with less significance when patients from Tranexamic acid for hyperacute primary intracerebral hemorrhage (TICH-2) were excluded6 (41.5 vs 45.0% in the control group; OR 0.87; 95% CI 0.57 to 1.34; p=0.52) (figure 1A). For patients with the spot sign, the rate of haematoma expansion was similar between the haemostatic therapy group and the control group (OR 0.98; 95% CI 0.60 to 1.59; p=0.93). For patients with the blend sign, the rate of haematoma expansion was marginally lower in the haemostatic therapy group with no statistical significance (OR 0.75; 95% CI 0.50 to 1.11; p=0.15). For patients with the black hole sign, a significant reduction of haematoma expansion was seen in the haemostatic therapy group (OR 0.61; 95% CI 0.39 to 0.94; p=0.03). The subgroup analysis on different haemostatic agents revealed no significant difference in the rate of haematoma expansion. Notably, there was no significant heterogeneity across the four studies and subgroups, except for the subgroup with the black hole sign (figure 1B,C).
Haematoma expansion for haemostatic therapy and placebo. (A) Analysis of all trials with and without TICH-2. (B) Subgroup analysis of CT signs. (C) Subgroup analysis of haemostatic agents. rFVIIa,recombinant activated coagulation factor VII; STOP-AUST,the Spot sign and Tranexamic acid On Preventing ICH growth—AUStralasia Trial; SPOTLIGHT,The“Spot Sign” Selection of Intracerebral Hemorrhage to Guide Hemostatic Therapy; STOP-IT,The Spot Sign for Predicting and Treating ICH Growth Study; TICH-2,Tranexamic acid for hyperacute primary intracerebral hemorrhage; TRAIGE,Tranexamic Acid for Acute ICH Growth prEdicted by Spot Sign.
Haemostatic therapy and clinical outcome
A total of four studies were included for the analysis of the clinical outcome. There was no significant heterogeneity between the four studies and their subgroups. When all patients were analysed, haemostatic therapy did not reduce the rate of the poor functional outcome when compared with placebo (53.3 vs 53.3% in the control group; OR 1.00; 95% CI 0.86 to 1.17; p=0.96 and 45.8 vs 46.3% in the control group; OR 0.94; 95% CI 0.60 to 1.47; p=0.78) whether TICH-2 (figure 2A) was included or not. The results in all subgroup analyses were generally consistent with the main analysis (figure 2B,C). No benefit was seen in reducing 90-day mortality after haemostatic therapy (figure 3A–C) in the main or subgroup analysis.
Poor functional outcome (mRS>3) for haemostatic therapy and placebo. (A) Analysis of all trials with and without TICH-2. (B) Subgroup analysis of CT signs. (C) Subgroup analysis of haemostatic agents. rFVIIa,recombinant activated coagulation factor VII; STOP-AUST,the Spot sign and Tranexamic acid On Preventing ICH growth—AUStralasia Trial; SPOTLIGHT,The“Spot Sign” Selection of Intracerebral Hemorrhage to Guide Hemostatic Therapy; STOP-IT,The Spot Sign for Predicting and Treating ICH Growth Study; TICH-2,Tranexamic acid for hyperacute primary intracerebral hemorrhage; TRAIGE, Tranexamic Acid for Acute ICH GrowthprEdicted by Spot Sign.
All-cause mortality for haemostatic therapy and placebo. (A) Analysis of all trials with and without TICH-2. (B) Subgroup analysis of CT signs. (C) Subgroup analysis of haemostatic agents. rFVIIa,recombinant activated coagulation factor VII; STOP-AUST,the Spot sign and Tranexamic acid On Preventing ICH growth—AUStralasia Trial; SPOTLIGHT,The“Spot Sign” Selection of Intracerebral Hemorrhage to Guide Hemostatic Therapy; STOP-IT,The Spot Sign for Predicting and Treating ICH Growth Study; TICH-2,Tranexamic acid for hyperacute primary intracerebral hemorrhage; TRAIGE, Tranexamic Acid for Acute ICH GrowthprEdicted by Spot Sign.
Sensitivity analyses and risk of bias
Sensitivity analysis for the rate of each endpoint showed that the overall effect of haemostatic therapy was consistent with the overall estimate from all studies excluding the TICH-2 study. Sensitivity analysis by sequentially dropping individual trials and then evaluating the overall outcomes failed to identify any of the individual trials that influenced the outcomes to any significant extent. Fixed-effects analyses showed a consistent trend for haemostatic therapy in all sensitivity analyses for the rate of haematoma expansion (online supplemental 4). There was also no significant publication bias detected with the examination of funnel plots for the outcome of haematoma expansion or with Egger’s regression test (online supplemental 5).
Supplementary data
Supplementary data
DISCUSSION
Our meta-analysis of meticulously performed RCTs that compared haemostatic therapy with placebo in patients with spontaneous ICH predicted by CT signs showed a potential nonstatistically significant benefit of reducing early haematoma expansion with haemostatic therapy. Haemostatic therapy did not lower the 90-day risk of poor functional outcome and all-cause mortality. However, in patients with the black hole sign, there was a statistically significant reduction of haematoma expansion with haemostatic therapy, a benefit not seen in subgroups with other CT signs. This is the first meta-analysis of published trials of high-quality and low bias risk that evaluates the effectiveness of haemostatic therapy for spontaneous ICH predicted by CT signs.
Haemostatic therapy for spontaneous ICH without CT signs
The rFVIIa was a rapid procoagulant developed for haemophilia-related haemorrhage. In previous trials (FAST-2,16 FAST-319), FVIIa reduced ICH expansion by about 50% compared with placebo but did not improve clinical outcomes.19 Tranexamic acid for patients with ICH was first tested in Malaysian trial (n=30)20 and TICH trial (n=24),21 which revealed a reduction of ICH expansion. Following that, a pragmatic phase III prospective double-blind randomised placebo-controlled trial, TICH-26 enrolled 2325 patients with ICH who received either tranexamic acid or placebo within 8 hours of onset. In the TICH-2 trial, tranexamic acid did not show any benefit in 90-day functional outcome when compared with placebo (adjusted OR 0·88; 95% CI 0·76 to 1·03; p=0·11), despite a reduction in early deaths and serious adverse events. However, a potential benefit of reducing haematoma expansion was seen in a smaller proportion of patients treated with the tranexamic acid (265 (25%)/1054 vs placebo 304 (29%)/1058) with an OR of 0·80 (95% CI 0·66 to 0·98; p=0·03). The absolute effect on reducing haematoma growth was modest (1 mL). Overall, haemostatic therapy had a slight benefit in reducing haematoma growth but did not significantly improve the functional outcome in patients with ICH without any CT signs.
Haemostatic therapy for spontaneous ICH with CT signs
Some researchers believe that haemostatic therapy is more appropriate for patients at high risk for ICH growth, such as patients presented early and with early CT signs predicting haematoma growth. For this reason, high-risk patients with ICH with image biomarkers, such as the spot, black hole and blend signs on CT, were identified as candidates for haemostatic therapy in recent randomised trials.
The Spot Sign for Predicting and Treating ICH Growth Study (STOP-IT) and the“Spot Sign” Selection of Intracerebral Hemorrhage to Guide Hemostatic Therapy (SPOTLIGHT), published in 2019, were phase 2 trials using the spot sign as a selection criterion.13 These trials recruited 69 patients with ICH over 6 years to receive either rFVIIa or placebo, with a median time from CT scan to treatment of 79 min (IQR 61–99) in the rFVIIa group. Both trials did not show any significant reductions of haematoma growth with rFVIIa (13 (41%)/vs 16 (43%)/37; p=0·83) or severe disability (9 (30%)/30 vs 13 (38%)/34; p=0·60).
The Spot sign and Tranexamic acid On Preventing ICH growth—AUStralasia Trial (STOP-AUST), published in 2020, was a phase 2 trial with 100 patients with ICH with the spot sign randomised to receive tranexamic acid (50) or placebo (50).14 The study did not show any benefit of tranexamic acid in reducing haematoma growth (OR 0·72; 95% CI 0·32 to 1·59; p=0·41), although the treatment is proven to be safe.
TRAIGE was a phase 2 trial that compared tranexamic acid with placebo in patients with ICH with the spot, blend or black hole signs.12 All qualified patients had an noncontrast computed tomography (NCCT) or contrast-enhanced CT within 6 hours from the onset. This latest study found no benefit of tranexamic acid on reducing haematoma growth (OR 0.96; 95% CI 0.52 to 1.77; p=0.89) or improving clinical outcomes with a generalised OR of 1.11 (95% CI 0.65 to 1.90; p=0.70), which is consistent with the previous studies.
Furthermore, in 249 patients enrolled in the TICH-2 trial, 56 patients with a positive spot sign were randomised to receive tranexamic acid (24) or placebo (32). The trial found a poor treatment effect of tranexamic acid in the subgroup analysis. In the post hoc analysis in the same study18 for the purpose of defining the role of the NCCT signs as predictors of haematoma expansion and poor functional outcome, blend sign, black hole sign and hypodensities were found to be predictive of haematoma expansion. Black hole sign, hypodensities and island signs predicted a poor functional outcome. The study did not show any significant correlation between the presence of signs and the benefit of tranexamic acid in reducing haematoma expansion. NCCT signs do not indicate a better response to tranexamic acid regarding the clinical outcome. In summary, studies on patients susceptible to haemorrhage expansion predicted by CT signs, especially the spot sign, showed that haemostatic therapy did not significantly prevent the haematoma growth or improve the outcome.
Future direction
This meta-analysis showed that haemostatic therapy might have the benefit of reducing early haematoma expansion in selected patients with ICH. It has been reported by a prior systematic review22 that haemostatic therapy does not increase the occurrence of thrombotic events. However, no significant improvement in prognosis or reduction in mortality was observed with haemostatic therapy in patients with ICH with or without CT signs. There are several possible explanations. First, modest absolute volume reduction in haematoma may not be sufficient to show clinical benefit.6 14 Second, the previous studies6 showed some other uncertainties that might impact the outcome. Haemostatic therapy could modestly reduce haematoma expansion but not enough to alter the pathophysiological process. For example, haemostatic therapy could not change perihaematomal brain oedema23 and other complications, similar to the limitations of surgical intervention for ICH.24 25 However, these unresolved clinical problems may have a bigger impact on the final prognosis of patients with ICH.23 Predictive CT signs used in these studies were proved to be valuable in predicting haematoma expansion and poor prognosis,26 but the underlying pathological mechanisms remain unclear. It appears that different CT signs may have different predictive roles in evaluating the therapeutic effects of the haemostatic therapy. It is time to evaluate different predictive CT signs in those subgroups that showed potentially improved outcome. In the post hoc analysis of Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH-2),27 ultraearly blood pressure (BP) reduction ≤2 hours could reduce haematoma growth and further improve functional outcome. Subgroup analyses from the TICH-2, STOP-AUST and TRAIGE all found that earlier treatment (within 2–3 hours) seemed to show more benefit. The proposed time window could also be used for haemostatic therapy in future research. More trials using tranexamic acid to treat ICH in the early time window are already ongoing (EudraCT 2012-005594-30 and ClinicalTrials.gov NCT03385928). There was speculation that haemostatic therapy might be similar to intravenous thrombolysis for ischaemic stroke, namely, earlier cessation of haematoma expansion might stop the trend of a cascade of deterioration.28
Even though the results of these studies do not support the haemostatic therapy at present, further studies on better-defined subgroups of ICH patients are warranted. Tranexamic acid has certain advantages for this role. It is low-cost, widely used, safe and suitable for promotion on a global scale. However, recruitment of patients with high-risk ICH is difficult because of the strict selection criteria and difficulty of obtaining emergency CT angiography routinely. Global collaboration may be the solution to the re-evaluation of tranexamic acid with a trial in a highly selective ICH population.
Limitation
The analysis on the overall population and subgroup population of TICH-2 were included in the current analysis, where the overall population also included patients without CT signs. To reduce the effect of bias of population differences, analysis with and without the overall population of TICH-2 was carried out separately as part of sensitivity analysis. However, the CI of primary analysis when patients from TICH-2 were excluded was significantly wider (p=0.52) than with TICH-2 included (p=0.051). Perhaps the sample size of the TICH-2 study is the main reason that TICH-2 may be driving much of the potential significance for reducing rate of haematoma expansion. The Factor VII phase IIb and phase III trials (FAST III) were representative researches on the evaluation of the effect of Factor VII on the prevention of haemorrhage growth in all ICH patients and the original data from FAST III on a subgroup of high-risk patients with ICH predicted by positive signs on CT could not be extracted from published reports, therefore, we could not get the original data. For these reasons, we excluded FAST III from the current analysis. Furthermore, subgroup analysis for each CT sign was performed to determine the difference in efficacy for patients with different CT signs. Since the inclusion of the RCT subgroup would lead to the decline of the overall study quality, the quality of subgroup analysis based on CT signs in this study was only equivalent to the level of a meta-analysis of cohort study. In addition, there were some differences in the protocols across the studies. For instance, the onset-to-randomisation time limits ranged from 4.5 to 8 hours, and different haemostatic agents were used. These differences may lead to differences in the results. Sensitivity analysis was carried out to test the interstudy bias, but no positive interstudy bias that affected the results was found. Moreover, there were obvious differences in the sample size across studies. The sample size of the TICH-2 study was significantly larger than that of other studies. However, there was no significant publication bias found.
CONCLUSIONS
Haemostatic therapy may reduce haematoma expansion at a marginally significant level but could not lower the risk of 90-day poor functional outcome and all-cause mortality in high-risk patients with ICH with predictive CT signs. Although the data did not support the wide use of haemostatic therapy clinically, it may have provided directions in future research of treating patients with ICH.
Data availability statement
Data are available upon reasonable request. Data in this article are available upon reasonable request.
Ethics statements
Acknowledgments
The authors thank Lina Zheng, Jiahui Zhao, Ying Tan, Yaozhi Chen and other study coordinators for their meticulous work and are grateful for the participation and engagement of all the subjects and investigators of the TRAIGE trial and this Meta-analysis.
Footnotes
Contributors Study concept and design: LL, SS and WW. Drafting of the manuscript: XN and JL. Statistical analysis: QZ and HG. Study supervision and organisation of the project: DL, HS and WD. Revision of the manuscript: YP, ZY and MW.
Funding The study is funded by grants from the Beijing Science and Technology Commission (D141100000114002), National Natural Science Foundation of China (81820108012, 81971614), National Key R&D Program of China (2016YFC1307301, 2018YFC1312402).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.