Abstract
Stem cells (SCs) are cells with strong proliferation ability, multilineage differentiation potential and self-renewal capacity. SC transplantation represents an important therapeutic advancement for the treatment strategy of neurological diseases, both in the preclinical experimental and clinical settings. Innovative and breakthrough SC labelling and tracking technologies are widely used to monitor the distribution and viability of transplanted cells non-invasively and longitudinally. Here we summarised the research progress of the main tracers, labelling methods and imaging technologies involved in current SC tracking technologies for various neurological diseases. Finally, the applications, challenges and unresolved problems of current SC tracing technologies were discussed.
Introduction
Stem cells (SCs) are undifferentiated cells of multicellular organisms, characterised by their multipotent differentiation and self-renewal capabilities.1 SCs can be divided into two types according to its source: embryonic SCs and adult stem cells (ASCs). ASCs currently found mainly include haematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), bone marrow mesenchymal stem cells (BM-MSCs), neural stem cells (NSCs), liver SCs, etc. When neuro-damage or degeneration occurs in the central nervous system (CNS), the repair of endogenous SCs is very limited, especially in neurons and glial cells. It brings broad prospects and new hope for SC transplantation treatment in neurological diseases such as stroke, Parkinson’s disease (PD), Alzheimer’s disease (AD), spinal cord injury (SCI), etc.2–7 After transplantation, SCs migrate to the injury site, differentiate and colonise to play a therapeutic role.8 Recently, a larger number of cell tracking methods in vivo were developed and applied in animals and humans, but the processes of SC migration, proliferation and differentiation are still not fully illustrated. State-of-the-art molecular and cell imaging techniques provide new and better means for non-invasive, repeated and quantitative tracking of SC therapy. Imaging technologies for in vivo cell tracking include MRI, nuclide imaging, optical imaging and multimodality imaging, which have their own advantages and disadvantages. The ideal method for SC labelling should be simple, easy to use, strong specificity, high sensitivity, no obvious toxicity, little external interference factors and low false-positive rate.9 10 In this review, the technology of SC labelling and tracking is reviewed and summarised, which provides a certain supporting role for the basic research and clinical translations of SC transplantation therapy.
Techniques for labelling and tracking SCs
Magnetic resonance imaging
MRI has unique capabilities in molecular and cellular imaging, providing high spatial resolution in vivo imaging.11 Especially in cell-based therapies, MRI non-invasively obtains spatial changes in cell-resolution anatomical positioning and has become an important method for tracking SCs in clinical SC therapy trials in neurological disorders.11
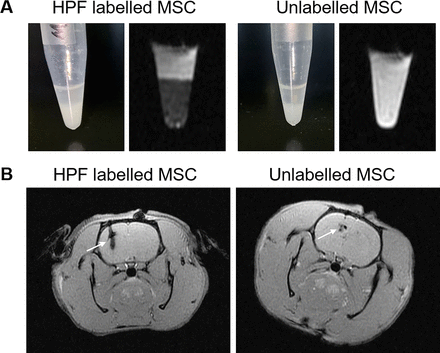
Paramagnetic substances used for MRI contrast agents are mainly gadolinium (Gd3+) and manganese (Mn2+), but both Gd3+ and Mn2+ are cytotoxic, which limits their application in vivo.12 Due to the superparamagnetic properties, iron-based agent outperforms conventional gadolinium-based MRI contrast agents. The contrast agent of heteronuclear magnetic resonance is mainly 19F, which is highly sensitive, and the signal intensity is directly proportional to the amount of 19F.13 Finally, the imaging reporter gene is to enhance the signal of MRI by introducing a certain gene into the target cell to make it express a certain substance. The current report genes used in MRI include lacZ, transferrin gene and ferritin gene.14 To visualise cells from the host cell background and increase their sensitivity, MRI-based cell imaging requires contrast agents to label the cells. Superparamagnetic iron oxide (SPIOs) is a kind of iron oxide nanoparticles exhibiting superparamagnetism. The high sensitivity in MRI allows it to use in very low concentrations, thereby reducing their side effects.15 16 SPIOs are usually coated with organic polymers to improve the stability and biocompatibility of the iron particles and further functionalise the particles.17 Ferumoxytol is currently the only SPIO contrast agent approved by the FDA.18 Recently, we combined ferulic acid, heparin and protamine into an ferumoxytol–heparin–protamine (HPF) nanocomplex to label and track MSCs by MRI.19 The HPF nanocomplex were prepared by sequentially adding heparin at 2 IU/mL, protamine at 60 µg/mL and ferumoxytol at 50 µg/mL in either sterile water for physiochemical characterisation or in serum-free RPMI-1640 medium for cell-culture studies (the timeline of HPF-labelled SCs is shown in figure 1). HPF-labelled MSCs were injected into the brain striatum of Wistar rats by stereotactic positioning, and MRI was performed on the third day after injection. Unlabelled MSCs were used as controls. As shown in figure 2, HPF-labelled MSCs show hypointensive signals in vitro through the T2*-weighted MRI (figure 2A). Similarly, in rats with HPF-labelled MSCs, we observed obvious hypointensive T2* signals on one side of the brain striatum on the third day (figure 2B). These data suggest that HPF nanocomplex combined with MRI can track and monitor MSCs. However, due to non-specific characteristics, these methods need relatively high SPIO nanoparticle concentrations and long incubation periods for proper labelling. Egawa et al proposed a specific and relatively fast method to label NSCs with SPIO nanoparticles via DNA hybridisation.20 Two short single-stranded DNAs, oligo(dT)20 and oligo(dA)20 were conjugated with a lipid molecule and SPIO nanoparticle, respectively. NSCs labelled with SPIO nanoparticles via DNA hybridisation system transplanted into the rat brain striatum were successfully detected by MRI in vitro as well as in vivo for up to 1 month. Overall, MRI is good at tracking SCs, although it has some drawbacks: non-specific imaging, the test object cannot contain magnetic substances and some of these contrast agents are generally slightly toxic.21
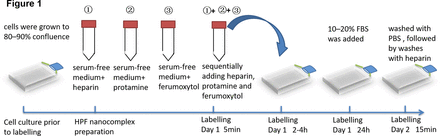
Timeline for HPF labelling MSCs. When the cultured MSCs grow to 80%–90% density, heparin, protamine and ferulic acid are added in sequence and mixed for 5 min. After 2–4 hours of cocultivation, 10%–20% FBS was added and the culture was continued for 24 hours. After washing with PBS and heparin, the culture was continued for 15 min to obtain HPF nanocomplex. HPF, ferumoxytol–heparin–protamine; MSC, mesenchymalstem cell;FBS, fatal bovine serun; PBS, phosphate buffered solution.
HPF nanocomplex combined with MRI can track and monitor MSCs. (A). The images show HPF-labelled and unlabelled MscS in the tube in vitro by the T2*-weighted MRI. (B.) the images show HPF-labelled and unlabelled MscS in the brain striatum of rats on the third day after stereotactic injection by the T2*-weighted MRI. HPF, ferumoxytol–heparin–protamine; MSC, mesenchymalstem cell.
Nuclear medicine imaging
Radionuclide-labelled cell imaging requires the application of gamma cameras, single-photon emission CT (SPECT) and positron emission tomography (PET).22 PET and SPECT are instruments with high sensitivity and high resolution in observing the distribution of cells in the body, which can determine the distribution and quantification of cells according to the ratio of the radioactivity of various tissues and organs.23
Nuclides can be imaged by two methods, one is direct labelling such as 18F; the second is to label specific cells with reporter genes that can import nucleotide sequence (reporter gene) that express specific phenotypic proteins (enzymes, receptors, transporters and so on) into the target cell gene.24 25 The expressed protein is then combined with specific radionuclide-labelled probes and detected by PET/SPECT, which indirectly reflects the distribution and quantification of target cells. The preferred radionuclides for PET are 18F, 11C, 13N and 15O, which have shorter half-lives.26 SPECT commonly uses radionuclides including 99mTc, 123 I67Ga and 111 In, with longer half-lives.27 28 Researchers need to pay attention to the leakage of the tracer, the half-life time and the survival time of labelled cells. In addition, the immune response caused by the expression product of the reporter gene should be considered.
Optical imaging
Optical imaging technology involves fluorescence imaging and bioluminescence imaging (BLI) .29 Fluorescence imaging uses fluorescent reporter genes such as enhanced green fluorescent protein (EGFP) for labelling. No substrate is needed, but excitation light is required to reach a higher energy level for the fluorophore and then emit longer-wavelength emission light.30
Cell lines stably expressed one or more luciferases or fluorescent proteins can be recognised and characterised as bioluminescent imaging tools.31 Fluorescent reporter gene imaging does not require substrates and can be used for both living and fixed cells and tissues.32 Both fluorescent and bioluminescence in vivo imaging have their limitations. In the visible light range, as photons are absorbed and scattered by the tissue, the emission signal attenuates approximately 10 times for each centimetre of tissue deepening.33
Multimodality imaging
The previously mentioned molecular and cellular imaging technologies have their unique advantages for the tracking and imaging of SCs, but when using only one of these imaging technologies, their inherent shortcomings cannot be ignored. One of the major challenges for tracking SCs is the neuroimaging resolution. The absolute resolution of MRI is 25–100 µm,34 and PET/SPECT is modest, around 1–2 mm.35Although considered less sensitive than PET/SPECT at the molecular level, MRI of ferromagnetic, paramagnetic or superparamagnetic markers can achieve substantially higher spatial resolution, depending on the magnetic field strength used, which can trace SC migration and differentiation, even neurite density.36–38 The absolute resolution of optical imaging is 2–5 mm.34 SC migration and differentiation can be traced by optical imaging with relatively low resolution, but increasing the resolution can limit the imaging depth substantially.36 39 Therefore, some studies have proposed a multimodal imaging mode, which combined two or more imaging modes. They have complementary advantages, overcome shortcomings of a single imaging mode and improve the sensitivity and resolution of tissue imaging, which is one of the research hotspots in recent years.40 For example, photoacoustic imaging (PAI) technology is one of the multimodal forms, combining optical imaging technology and ultrasonic imaging technology. At present, PET/CT (CT) and SPECT/CT have been widely used in clinical practice. The key to multimodality imaging lies in multifunctional contrast agents. In recent years, due to the rapid development of multifunctional contrast agents, multimodality imaging technology exhibit great potential in tracking SCs in vivo.
SC tracing in an animal model and clinical trials of neurological diseases
The application of SC-based therapies to treat neurodegenerative and traumatic injuries is gradually being discovered and recognised.41 However, the monitoring of cellular grafts, non-invasively, is an important aspect of the ongoing efficacy and safety assessment of SC-based therapies.42 Currently, 7840 clinical trials for SCs have been registered, of which about 480 are clinical trials for neurological diseases, but only a small number of clinical trials for SC transplantation use SC tracing.43 This limitation makes it difficult to confirm that the SCs have been delivered successfully to the target and even more difficult to track their progress over time. Overcoming these technological hurdles to develop a successful label is essential for advancing the field of SC transplantation. We identified 10 published articles that used different tracers to track the biological distribution of transplanted SCs in neurological diseases (table 1).
Labelling and tracking stem cells in neurological diseases
Stroke
Stroke is one of the leading causes of death and disability worldwide.44 Although acute treatment such as thrombolytic agents has been recommended, many patients have enduring deficits due to no efficient treatments for long-term recovery. SC therapy could arrange an alternative intervention for disease-modifying therapy. Several clinical trials of SCs therapies have been carried out, mainly for the treatment of chronic ischaemic stroke, and there are few studies on the treatment of acute and subacute ischaemic stroke. There are currently more than 80 clinical trials registered for SC treatment in stroke, of those clinical trials for NSCs and MSCs were clinical phase I and phase II.45–48 The ongoing phase III clinical trial of multipotent adult progenitor cells intended to recruit 300 patients and shorten the treatment time window (≤36 hours) to confirm whether the earlier intervention is effective for stroke recovery treatment.49 50
Study shows that MSCs can be labelled with SPIO nanoparticles (Molday ION-Rhodamine B) and catheter-based intra-arterial (IA) injected to the desired area of the CNS in large animals including pigs and dogs and small animals such as rats.51 Walczak et al confirm that high-speed MRI based on the GE-EPI pulse sequence can be used to monitor the SPIO-labelled SC of IA delivery to the CNS in real time.51 In addition, SPIO-based contrast agents can predict SC destinations and verify vascular patency after IA infusion of cells on large and small animals.
Dual-mode imaging has been pursued in recent years to track SCs in vivo. Limet al developed bicyclic nonyne (BCN) conjugated ethylene glycol chitosan nanoparticles (BCN-NPs) as dual-mode SC imaging probes.52 The near-infrared fluorescent (NIRF) dyeCy5.5 was coupled with BCN-NPs, and oleic acid-coated SPIOs (OA-Fe3O4 NPs) were encapsulated into Cy5.5-labelled BCN-NPs to generate OA-Fe3O4 NP-encapsulated BCN-NPs (BCN-dual-NP). They demonstrated that the use of the NIRF/T2-weighted dual-mode MRI can be used to effectively track the migration of BCN-dual-NPs-labelled human MSCs from the implantation site into the brain of mouse photothrombosis (PTS) models for 14 days. Compared with nanoparticle-only labelling technology, this bio-orthogonal labelling of human MSCs can greatly improve cell labelling efficiency, safety and imaging sensitivity. Chen et al successfully developed a multifunctional nanoseaurchin probe, in which iron oxide nanoparticles were embedded and multi-gold nanorods (multi-GNRs) crystal-seeded magnetic mesoporous silica nanobeads, and the umbilical cord MSCs was labelled by endocytosis.53 With dual modes combining PAI and MRI, MSCs can be successfully monitored in vivo for a long time in stroke mice.53 Zhang et al constructed a lentiviral vector encoding ferritin heavy chain (FTH) and EGFP (LV-FTH-EGFP) to deliver the reporter gene to NSC.54 The distribution and migration of transplanted NSCs in MCAO mice were successfully monitored by bimodal MRI and fluorescence imaging (FLI) for 6 weeks.
When dual-mode imaging cannot meet the requirements of the research, MRI/SPECT/fluorescent tri-modal probe was synthesised by labelling fluorescent silica-coated SPIOs with 125iodine (125I-fSiO4@SPIOs) for quantitatively tracking MSC transplantation into stroke rats.55 They demonstrated that 125I-fSiO4@SPIOs have high efficiency for labelling MSCs without affecting their viability, differentiation and proliferation capacity, thus is considered a robust probe for long-term MSC tracking in ischaemic rats.
Neurodegenerative diseases
The prevalence of neurodegenerative disorders is increasing, partly owing to extensions in lifespan, but effective treatments are still lacking. Neurodegenerative diseases are caused by the loss of neurons and /or myelin sheaths, which worsen over time and become dysfunctional, such as PD, AD and amyotrophic lateral sclerosis (ALS). While the pathological mechanisms of these neurodegenerative diseases are clear, attempts to change the course of the disease have all been unsuccessful.
The goal of SCs therapies in PD is to replace nigrostriatal dopaminergic neurons, which have been shown to improve motor symptoms such as stiffness, poor movement, tremors and instability.56 NSC and BM-MSC transplantation for PD is in the phase I/II clinical trial stage.57–59 American International Stem Cell Corporation announced the interim results of the first clinical cohort study of human parthenogenetic NSC line ISC-hpNSCs for PD.60 After 6 months of cell transplantation, the safety of patients with PD has been guaranteed and symptoms improved.60 But there are no reports of clinical and long-term effects of BM-MSCs, and patients need to continue to be recruited for long-term observation and follow-up. Immortalised human NSCs derived from VM (hVM1 cell line) are powerful research tools and potentials for cell therapies for PD. Ramos-Gomez et al tracked magnetic nanoparticles (MNPs)-labelled immortalised human NSCs for 5 months after implantation into the affected sites of the rat brain without showing significant neuronal or systemic toxicity or behavioural changes by MRI.10
Out of the wide range of neurodegenerative diseases, SC application seems to be particularly appealing for AD.61–63 In the last decade, although there are more evidence of SCs therapies in mammals, clinical trials of SCs therapies for AD are relatively few.64 The phase I/II clinical trials based on SCs have been conducted and still in their early stages,65 and no serious adverse events occurred during the follow-up period, paving the way for further efficacy and clinical benefit studies.66 Lee et al labelled human umbilical cord blood MSCs (hUCB-MSCs) with the HPF nanocomplex we described earlier and implanted them into the hippocampus of a transgenic mouse model of familial AD (5XFAD) by stereotactic injection and monitored by MRI. They confirmed that ferumoxytol can monitor transplanted MSCs in real time and is non-toxic to the viability of hUCB-MSCs.67
ALS is a fatal neurodegenerative disease characterised by the progressive degradation of motor neurons (MNs) in the brain and spinal cord, leading to muscle weakness, paralysis and death. SCs have potential advantages in treating ALS. Transplanted SCs can prevent the degradation of existing MNs by releasing neuroprotective nutrition factors and regulating immunity, and change the toxic microenvironment of ALS.68 Although single-armed, phase I/II clinical trials found that SC-based therapy for ALS is relatively safe and feasible, it is uncertain whether SC transplantation may be clinically beneficial leading to functional improvements and of the slowing of disease progression.69–71 The number of patients involved in the SC transplantation research of the clinical stage is limited, and it is difficult to obtain a more accurate statistical basis. Richard et al implanted human glial-restricted progenitor (hGRP) cells labelled with a perfluorocarbon (PFC) dual-modal tracer into the spinal cord of mouse models of ALS.72 This study demonstrates the feasibility of incorporating PFC into hGRPs for in vivo MRI.
Traumatic brain injury
Traumatic brain injury (TBI) is mainly an irreversible neuron injury that is common in the nervous system. TBI causes severe brain injury and difficult to recover physiological functions in the later stage. Currently, there is no effective treatment and SC transplant brings new hope to TBI.73 74 Preclinical research in small animal models of TBI has paved the way for early phase I/II clinical trials of SCs therapies.75 Nevertheless, significant barriers remain to conducting randomised controlled trials for efficacy.76 The optimal SCs type, dose, delivery route and timing of administration would be necessary and for how long needs to be determined in humans.76 Induced pluripotent SCs (iPS cells) are the most promising cell sources for NSCs.77 Jiang et al implanted SPIOs-labelled iPS cell-induced NSC into TBI rats.78 A manganese-enhanced MRI (ME-MRI) scan successfully monitored that iPS cell-induced NSCs can migrate from the injection site to the injured brain area within 1 month after implantation.
Spinal cord injury
The incidence of SCI is increasing year by year with a high disability rate.79 SC transplantation for the treatment of SCI makes it possible to regenerate injured axons, rebuild synapses and restore some functions of the spinal cord. Promising results have been obtained in the preclinical setting and in establishing basic safety data in clinical trials, but the translation of SCs to the clinic is still in its infancy.80 Therefore, SCs therapies have not been approved for SCI.81 82 Most clinical trials transplanting SCs into the spinal cord have not incorporated a method for tracking cells in vivo.83–85 Zhang et al labelled BM-MSCs with neurofilament-200 promoter and lipase-activated nanoparticle-containing nanoparticles (gadolinium-dimethylene penta-acetic acid-containing nanoparticles, Gd-DTPA-FA).86 Double-labelled bone marrow stromal SCs were implanted into the SCI model rats for MRI, diffusion tensor imaging and CT imaging for 28 days. Their research shows that the migration and distribution of Gd-DTPA-FA labelled BM-MSCs can be tracked by MRI.
Conclusion
The emergence of SCs has brought hope for the treatment of many incurable neurological diseases, but its clinical application is still limited. When we tried to integrate the researches from different groups, the biggest problem was the diversity of application methods and cell sources. Even if SCs of the same source are isolated by the same method, their phenotypes and biological characteristics are different. Finding the best SC line for clinical use is challenging.
Current SC tracing studies have established multiple imaging technologies that allow people to monitor the survival and migration of transplanted SCs in vivo. Researchers can choose the appropriate imaging technology based on factors such as the detection sensitivity, temporal and spatial resolution, imaging depth, security, labelling method, research purpose and object of different in vivo imaging technologies. Present imaging technologies have their own advantages and disadvantages. In view of the limitations of current imaging technologies, it is necessary to further improve the existing technologies and develop new imaging technologies. Establishing a convenient, fast, high-throughput and quantifiable SC tracking technology to meet the needs of fast and high-throughput screening is an urgent need. Among these techniques, the combined NIR/BLI imaging technology and quantitative methods with higher tissue penetration depth and spatial resolution will be one of the most effective ways. In addition, establishing a safe non-reporter method that can clinically monitor transplanted SCs and evaluate the efficacy of SCs is urgent and more challenging. Constructing MRI and PET probes that respond to cell death and viability, combined with high sensitivity and high tissue penetration depth-combined PET/MRI imaging technology is currently the most promising for clinical applications. Future research should balance between the best tracer effect and the cytotoxicity of the tracer, in the development of new tracers. In addition, with the deepening of life science research, more efficient cell tracking technology will be combined with single-cell genomics to open a new chapter in cell biology and developmental biology research and have a profound impact on clinical disease mechanisms and cell therapy research.
At present, several special and landmark tracking technologies as described earlier need to be further studied, which will help understand SC migration pathways, survival time, interactions and improve the tissue microenvironment. In the future, development of more ideal SC tracking technology will definitely help to accelerate the clinical transformation application of SC therapy.
Footnotes
XY and D-CT are joint first authors.
Contributors XM and W-NJ formulated the study concept and designed the studies; XY and DT wrote and edited the manuscript; WH, WL, JF and HL assisted preparation of the manuscript.
Funding This study was funded by National Science Foundation of China (81801199, 81830038, 81971094, 91949208), Beijing Municipal Science & Technology Commission (Z181100001818001).
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.