Abstract
Background We aim to comprehensively assess and compare the predictive performance of haematoma expansion (HE) scores in a homogeneous cohort of acute intracerebral haemorrhage (ICH) patients.
Methods Existing scores for predicting HE in acute ICH patients were included and categorised by imaging modality: non-contrast CT (NCCT), single-phase CT angiography (sCTA) and multiphase CTA (mCTA). The predictive performance of the scores was evaluated with the c-statistic in a population of consecutive adult patients with acute ICH admitted to a tertiary care centre in Southern Alberta, Canada, between February 2012 and May 2020, investigated with a multimodal imaging protocol (NCCT, sCTA and mCTA). The primary outcome was HE (ICH volume growth ≥6 mL or ≥33%), and the secondary outcome was severe HE (ICH volume growth ≥12.5 mL or ≥66%). The DeLong test compared the best-performing scores from each imaging category.
Results 16 HE scores were assessed (NCCT=8, sCTA=6 and mCTA=2) in 217 patients with a median age of 70 years (IQR=60–80), and 86 (39.6%) were females. 51 (23.5%) patients experienced HE and 35 (16.1%) had severe HE. The c-statistic for predicting HE ranged from 0.516 to 0.674 for NCCT-based scores, 0.627 to 0.725 for sCTA-based scores and 0.800 to 0.814 for mCTA-based score. The c-statistic for predicting severe HE ranged from 0.505 to 0.666 for NCCT scores, 0.651 to 0.740 for sCTA scores and 0.813 to 0.828 for mCTA scores. A statistically significant difference favouring mCTA over other imaging modalities in predicting both HE and severe HE was observed.
Conclusions Advanced imaging demonstrated a stepwise improvement in the predictive performance of HE scores. However, no existing score achieved excellent predictive performance (c-statistics ≥0.90) in our cohort, highlighting the need for further refinement.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Several haematoma expansion scores have been developed, yet they have not been compared in a homogeneous, unselected population.
WHAT THIS STUDY ADDS
Advanced imaging demonstrated a stepwise improvement in the predictive performance of haematoma expansion scores (mmultiphase CT Aangiography>ssingle-phase CT Aangiography>NCnon-contrast CT).
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Future research should focus on developing and validating new scores that leverage nuanced features of the spot sign to inform acute treatment decision-making.
Introduction
Intracerebral haemorrhage (ICH) is a devastating condition characterised by high mortality rates and significant disability.1 Haematoma expansion (HE) occurs in 20%–30% of cases within the first hours and is associated with poorer clinical outcomes, with estimated odds of severe disability or death increasing by 5% for every 1 mL of haematoma growth.2 3 Prevention or mitigation of HE is needed with approaches such as haemostatic treatment, anticoagulation reversal, minimal surgical intervention and blood pressure control.2 4 However, when applied broadly to an unselected ICH patient population, these treatments have shown limited effectiveness and have been hampered by potential adverse effects in clinical trials.4 5 Future trials and treatment protocols require better ways to predict the occurrence and severity of HE to aid in optimal patient selection. Predictors of HE can be broadly categorised into (1) clinical (ie, time from symptom onset, use of antithrombotic and clinical severity) and (2) imaging-based features. For practical reasons, imaging is done mostly with CT. Within this category, several imaging modalities can be applied: (a) non-contrast CT (NCCT) (ie, initial haematoma volume; density and shapes heterogeneity signs), (b) single-phase CT angiography (sCTA) (ie, presence and number of spot sign; colocalisation with hypodensity) and (c) multiphase/dynamic CTA (mCTA) (ie, time of first appearance and evolution of spot sign).2 6–9 Multiple scores integrating several of these factors have been developed, yielding a predictive c-static ranging from 0.62 to 0.93, with scores including advanced imaging features consistently showing superior performances.10–12 However, these scores have been explored in heterogeneous populations, using different definitions of HE, focusing solely on haematoma occurrence and not its severity, and some lack validation, making direct comparisons of their performance challenging.
Aim and hypothesis
We aim to comprehensively assess and compare the predictive performance of multiple HE scores in a homogeneous single-centre cohort of acute ICH patients investigated with a multimodal CT imaging protocol.
Methods
Search strategy and selection of HE scores
We included scores predicting HE in adults with non-traumatic primary ICH. We excluded (1) scores developed in small samples (<100 patients), (2) nomograms that could not be calculated by a simple scoring sheet but required a visual tool to map points, (3) studies with only predictive markers that did not develop scores/scales and (4) studies exploring radiomics and machine-learning predictors. Scores were selected from a previous systematic analysis by Yogendrakumar et al (inception to June 2018)10 and an updated literature review (June 2018 to May 2024). For the updated literature review, we searched PubMed and Scopus and screened all retrivied titles using the following MeSh term: (1) Predict, hematoma expansion score* or scale, intracerebral hemorrhage; (2) Predict, hematoma expansion score* or scale*, intracerebral hemorrhage, Hematoma growth and (3) Predict, hematoma expansion score* or scale*, intracerebral hemorrhage, hematoma growth, CT angiogram.
The HE scores were categorised based on the presence of NCCT, sCTA and mCTA imaging features. In cases where multimodal imaging features were considered in the score, it was categorised based on the most advanced imaging technique (ie, in the case of NCCT and sCTA variables, the score was classified as a sCTA score).
Patient sample
This retrospective, single-centre study involved consecutive adult patients with acute ICH admitted to a tertiary care centre in Southern Alberta, Canada, which caters to approximately 2 million people, between February 2012 and May 2020. The inclusion criteria were (1) time from symptom onset to imaging <12 hours, (2) acquisition of NCCT, sCTA and mCTA and (3) follow-up neuroimaging (CT or MRI) performed between 12 and 72 hours after baseline imaging. Patients were excluded if they had (1) a secondary ICH aetiology, (2) isolated intraventricular haemorrhage (IVH) or (3) underwent neurosurgical intervention before follow-up imaging. Acute ICH management followed local protocol and guidelines.
Baseline clinical, laboratory and radiological variables
The baseline clinical variables collected included onset-to-imaging time, ultra-early haematoma growth (initial ICH volume/hour from the onset-to-imaging time), history of previous haemorrhagic stroke, anticoagulation use, antiplatelet use, baseline National Institutes of Health Stroke Scale (NIHSS) and baseline Glasgow Coma Scale. The baseline laboratory variables collected included international normalised ratio and serum glucose levels. For patients with unknown onset, the last time known well was considered the onset time to calculate both onset-to-imaging time and ultra-early growth. All anticoagulant drugs (vitamin K antagonists, direct oral anticoagulants or heparin) were considered when assigning point(s) for the variable ‘anticoagulant at the onset’.
The baseline NCCT radiological variables collected included initial ICH volume, hypodensity sign, blend sign, fluid-level sign, swirl sign, heterogeneous density, irregular shape, island sign and extension to the ventricular system, that is, IVH.6 The ‘niveau’ formation was defined as either the presence of a blend sign or a fluid-level sign.13 The CTA radiological variables collected included the presence of spot signs, their phase of appearance, the maximum density of spot signs (maximum Hounsfield unit), the maximum diameter of spot signs, and the number of spot signs.14
Image acquisition and analysis
All patients received a comprehensive acute stroke imaging protocol, which included NCCT and multiphase CTA using either the Discovery CT 750HD or Revolution CT (GE Healthcare, Milwaukee, Wisconsin, USA). The CTA was conducted with a bolus-tracking technique involving the injection of 70 mL of non-ionic iodinated contrast (68% ioversol, Optiray 320, Mallinckrodt, St Louis, Missouri, USA) at a rate of 6 mL/s. The mCTA ICH protocol included three phases with 10–11 s between the first and second phases and a 16–18 s time interval between the second and third phases.14 The first phase of the mCTA protocol covered the entire cervical and intracranial vasculature (from aortic arch to vertex) and represented a routine sCTA acquisition. The second and third phases covered the intracranial vasculature from the skull base to the vertex.15 Axial images were acquired with a section thickness of 0.625 mm and reconstructed with a 1 mm overlapping section. Scanning parameters included a tube voltage of 120 kV and an auto tube current ranging from 10 to 625 mA. Haematoma volumes at baseline (initial volume) and follow-up (final volume) were calculated with the semiautomatic segmentation software Quantomo (Cybertrial, Calgary, Canada).16 The total ICH volume included both intraparenchymal and intraventricular components.
NCCT shape and density signs were collected according to the international NCCT collaboration group standards.6 The CTA spot sign was defined as a serpiginous or spot-like appearance of contrast within the margins of the ICH (1) without connection to a vessel, (2) a maximum diameter ≥1.5 mm, (3) a contrast density double the background haematoma or ≥120 Hounsfield unit and (4) no hyperdensity at the corresponding NCCT.17 NCCT and CTA markers were read by two neurologists and stroke imaging researchers (UP and KT) with 4 and 16 years of experience, respectively, blinded to clinical and radiological outcomes.
Outcomes
The primary outcome was HE, defined as ICH volume growth ≥6 mL or ≥33% between the initial and final volumes. The secondary outcome was severe HE (sHE) defined as ICH volume growth ≥12.5 mL or ≥66%.18
Statistical analysis
Continuous variables were presented as medians and IQRs, while categorical variables were reported as counts and percentages. Baseline clinical and radiological features, as well as clinical and radiological outcomes, were compared between patients who experienced HE and patients who did not. Univariable comparisons were conducted using Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. Discrimination of the HE scores for outcomes was evaluated with receiver operating characteristic curves, reporting the area under the curve (c-statistic) with 95% CIs, along with the Aikake information criterion and Bayesian information criterion. Calibration plots and the Brier score were used to assess the calibration of the predictive scores. The Brier score was calculated as the mean squared error between the predicted probabilities and the observed outcomes, ranging from 0 to 1, where 0 indicates perfect calibration. The DeLong test was used to compare the best-performing scores from each imaging category. No inter-reader or intrareader agreement was assessed for radiological expansion markers or expansion scores. All calculated p values were two-tailed. Statistical significance was assumed at p<0.05. The statistical analysis was performed with Stata (V.18.0).
Results
Included NCCT, sCTA and mCTA scores for HE
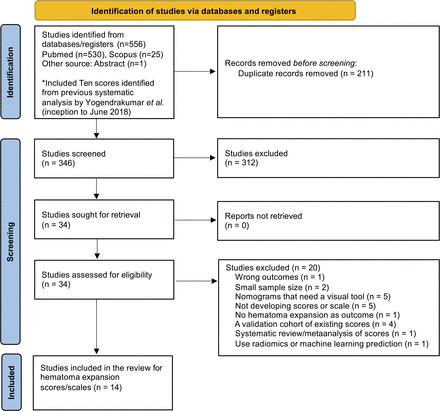
Overall, 16 HE scores (NCCT scores=8, sCTA scores=6 and mCTA scores=2) from 14 studies were selected to be explored and compared in our cohort (figure 1):
NCCT scores: (1) BAT (Blend sign, Any hypodensity, Time) Score,19 (2) BRAIN (Baseline ICH volume, Recurrent ICH, Anticoagulation, Intraventricular extension, Number of hours to baseline CT from symptom onset) Score,8 (3) Basal Ganglia Score,20 (4) NAG (NIHSS, Anticoagulation, Glucose) Score,21 (5) HEAVN (Heterogeneity, peripheral Oedema, Anticoagulant use, Volume, Niveau formation) Score,13 (6) NCCT expansion Score,11 (7) ‘Li et al NCCT Score’,22 (8) ‘Kong et al NCCT Score’.23
sCTA scores: (1) ‘applied to sCTA-Spot Sign Score’,24 (2) 9-Point Score,25 (3) PREDICT A Score,26 (4) PREDICT B Score,26 (5) Acute ICH Growth Score and27 (6) sCTA expansion Score.11
mCTA scores: (1) ‘applied to mCTA- Spot Sign Score’ and24 (2) mCTA expansion Score.11
Flow chart of literature review and reasons for exclusion. Sixteen scores were derived from the final 14 studies. *Included the Spot Sign Score, 9-Point, BRAIN, PREDICT A/B (Prediction of Haematoma Growth and Outcome in Patients With Intracerebral Haemorrhage Using the CT-Angiography Spot Sign), HEP (Haematoma Expansion Prediction), Acute ICH Growth Score, HEAVN, BAT, Basal Ganglia Score and NAG Score. BAT, Blend sign, Any hypodensity, Time; HEAVN, Heterogeneity, peripheral Oedema, Anticoagulant use, Volume, Niveau formation; ICH, intracerebral haemorrhage; NAG, NIHSS, Anticoagulation, Glucose; NIHSS, National Institutes of Health Stroke Scale.
Details of the included variables and the calculation method for individual scores can be found in the online supplemental fmaterials and online supplemental tables 1–3.
Supplementary data
Patient characteristics
217 patients were included in the study, the median age was 70 years (IQR=60–80), 86 (39.6%) were females and 39 (18.0%) were taking anticoagulant drugs. The median baseline NIHSS was 10 (IQR=5–20), the median initial ICH volume was 18.9 mL (IQR=5.5–34.2) and the median onset-to-imaging time was 225 min (IQR=109–392). HE and sHE were seen in 51 (23.5%) and 35 (16.1%) patients, respectively. Detailed demographics, medical history, baseline features, radiological markers of expansion and radiological outcomes of the study cohort are summarised in table 1.
Patient characteristics
Prediction accuracy of NCCT, sCTA and mCTA scores for HE and sHE
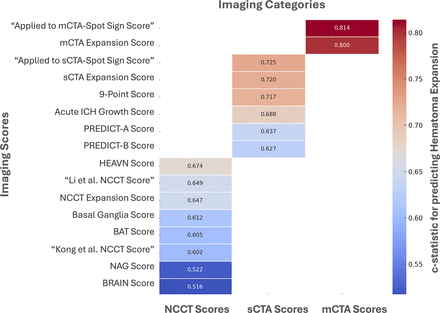
The c-statistic for predicting HE ranged from 0.516 to 0.674 for NCCT scores (table 2), 0.627 to 0.725 for sCTA scores (table 3) and 0.800 to 0.814 for mCTA scores (table 4, figure 2). The c-statistic for predicting sHE ranged from 0.505 to 0.666 for NCCT scores (table 2), 0.651 to 0.740 for sCTA scores (table 3) and 0.813 to 0.828 for mCTA scores (table 4).
Discrimination of non-contrast CT (NCCT) Scores to predict haematoma expansion
Discrimination of sCTA scores to predict haematoma expansion
Discrimination of mCTA Scores to predict haematoma expansion
The heatmap compares the performance (measured as c-statistics) of various predictive scores for intracerebral haematoma expansion (≥6 mL or ≥33%) grouped by imaging modality, including non-contrast CT (NCCT), single-phase CT angiography (sCTA) and multiphase CT angiography (mCTA). The colour gradient highlights the relative differences in performance, with darker colour indicating high c-statistics.
Calibration plots are shown in online supplemental figure 1–16.
The comparison of the best-performing scores across each imaging category showed a statistically significant difference favouring mCTA over other imaging modalities in predicting both HE (mCTA vs NCCT, p=0.006; mCTA vs sCTA, p=0.003) and sHE (mCTA vs NCCT, p=0.001; mCTA vs sCTA, p=0.013) (table 5).
Results of the DeLong test for pairwise comparison of c-statistics of the best performing score for each imaging category
Discussion
We comprehensively compared 16 HE scores in a homogeneous patient population, demonstrating a stepwise improvement in predictive performance with the incorporation of advanced imaging protocols. Specifically, the c-statistic for predicting HE improved by approximately 0.5 points with sCTA imaging and by approximately 1.4 points with mCTA imaging compared with scores that relied solely on NCCT markers. A similar trend was observed when assessing sHE.
In our cohort, NCCT HE scores exhibited a wide range of performance, with c-statistics ranging from 0.516 to 0.674. The HEAVN Score13 and ‘Li et al NCCT Score’22 demonstrated the highest predictive performance. Our results are consistent with previous studies showing good performances of these two scores in their respective validation cohorts (c-statistics ≥0.80).13 22 Nonetheless, none of the eight scores tested achieved a c-statistic above 0.7 in our cohort, highlighting their limitations in predicting HE without advanced imaging support.
In our cohort, sCTA scores demonstrated a c-statistic ranging from 0.627 to 0.725. The ‘applied to sCTA-Spot Sign Score’24 showed the highest predictive performance. This score focuses solely on the presence of the spot sign and its characteristics (number, maximum diameter and maximum CT density).24 In contrast, the second-best-performing score was the sCTA Expansion Score,11 which incorporated the presence of the spot sign with an estimate of pre-scan haematoma growth. This suggests that combining spot sign nuances with time-dependent factors might significantly improve the predictive accuracy. Notably, the ‘applied to sCTA-Spot Sign Score’ is the only sCTA score that achieved a C-statistic higher than 0.9 in a validation study,28 yet these results were not confirmed in another validation study.10
In our cohort, the two mCTA scores demonstrated the highest predictive performance, with c-statistics of 0.800 and 0.814. The best-performing score was the ‘applied to mCTA-Spot Sign Score’24 which evaluates the morphological features of the spot sign, but not its temporal evolution.
Overall, the c-statistics for the scores were lower than those reported in the original studies. This is consistent with the trend observed in validation studies, which typically demonstrated reduced predictive performance compared with the development studies (most scores were assessed only in development cohorts). Additionally, the shorter time from symptom onset to imaging in the original studies compared with our cohort—both in development and validation cohorts—might suggest that these scores are more effective within the early time window.10
Our findings demonstrated a remarkable progressive improvement in the predictive performance of the scores with the use of more advanced imaging, which is in line with results from a previous single-centre study.24 Nonetheless, none of the scores achieved excellent discrimination (c-statistic ≥0.90), even with mCTA imaging. However, emerging evidence has shown additional nuances of the spot sign that can significantly increase the prediction accuracy for HE occurrence and severity.7 9 14 29–32 While some dynamic features have been incorporated in previous scores, such as the time of first appearance, other pivotal characteristics have not. Specifically, changes in the spot sign volume over time, whether an increase or decrease, have shown high predictive value.14 33 Another recently described aspect is the colocalisation of the spot sign with a hypodensity sign on NCCT (‘black-and-white sign’). This colocalisation presumably represents a site of long-lasting bleeding or impaired haemostasis (hypodensity) that continues to bleed (spot sign).9 34
Improved risk stratification for HE is urgently warranted as our armamentarium of potentially effective anti-HE interventions expands. Indeed, hyperacute interventions that have demonstrated clinical benefits carry non-negligible risks, such as thromboembolic complications with reversal anticoagulant therapy, acute kidney injury with intensive blood pressure lowering and surgery-related risks.35–37 Despite advancements in our understanding of HE, scores have not been employed in clinical practice—unlike in other areas of cardiovascular medicine, where scoring systems guide decision-making in balancing the risks and benefits of interventions (e.g., the CHA2DS2-VASc Score).38 A novel scoring system that integrates nuanced features of the spot sign holds promise to finally achieve consistently a c-statistic above 0.90 for predicting HE, with the potential to influence acute decision-making significantly. An accurate stratification of the risk of HE occurrence and severity might tailor the timing, intensity and type of hyperacute interventions. A potential limitation of incorporating nuances of the spot sign, such as volume changes, into a HE score for clinical practice is the challenge of assessing these nuances in the acute setting. However, automated software, particularly those driven by artificial intelligence, could streamline this process and deliver real-time scores.
Our study has several limitations. First, it involved a relatively small, single-centre cohort, thus limiting the generalisability of our findings. Nonetheless, it remains one of the largest cohorts to include mCTA imaging, enabling a comprehensive comparison of all described scores. Second, we excluded patients who underwent surgical haematoma evacuation prior to follow-up imaging, which may have led to preferential exclusion of patients with the highest risk of HE, possibly resulting in a slightly biased study sample. Third, the timing and type of hyperacute treatment administered in the emergency room might influence HE occurrence and severity, yet it was not systematically collected in our cohort. Finally, we could not assess two specific scores due to missing data. Yet, these scores demonstrated only modest performance in their original development cohorts. Despite this, our study validated several scores that had previously lacked external validation.
Conclusions
Advanced imaging demonstrated a stepwise improvement in the predictive performance of HE scores. However, no existing score achieved excellent discrimination in our cohort, highlighting the need for further refinement. Future research should focus on developing and validating new scores that leverage nuanced features of the spot sign to inform decision-making of anti-HE therapeutic interventions. Furthermore, quick, automated software solutions for ICH assessment, including automated calculation of imaging-based risk stratification scores, should be a high research priority to allow broad use of these scores in clinical routine.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and the ethics committee at the University of Calgary approved the study (approval protocol number: REB17-0573). In accordance with ethical guidelines, informed consent was waived for this study due to its retrospective nature.
Acknowledgments
We would like to thank the following individuals, who are part of the MCAHP Stroke Group, for their contribution to the study: Ericka Teleg, Abdulaziz Sulaiman Al Sultan, Linda Kasickova, Tomoyuki Ohara, Piyush Ojha, Sina Marzoughi, Girish Kulkarni, Dar Dowlatshahi.
Footnotes
X @UPensato
Contributors UP drafted the manuscript and performed the statistical analysis. UP, KT and AD conceived the study. UP, KT, CK, JO, MH, DR-L, NS, AB, SW, KH, AB, AN, WQ, MG, BM and AD collected the data and reviewed the manuscript for content. AD supervised the study and is the guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.