Abstract
Background Despite successful reperfusion after thrombectomy for large vessel occlusion (LVO) stroke, up to half of patients are dependent or dead at 3-month follow-up.
The aim of the current study is to demonstrate safety and efficacy of administering adjunct intra-arterial (IA) tenecteplase in anterior circulation LVO patients who have achieved successful reperfusion defined as eTICI 2b50 to 3.
Methods ANGEL-TNK is a multicentre, open-label, assessor-blinded endpoint, prospective randomised, controlled trial that will enrol up to 256 patients. Patients who meet inclusion criteria with anterior circulation LVO stroke and successful reperfusion will be randomised to receive IA tenecteplase or best medical management at 1:1 ratio.
Results The primary endpoint is a 90-day excellent outcome defined as modified Rankin Scale (mRS) 0–1. The primary safety endpoint is symptomatic intracranial haemorrhage within 48 hours from randomisation. Secondary endpoints include 90-day ordinal mRS, mRS 0–2, mRS 0–3, all-cause mortality and any intracranial haemorrhage.
Conclusion In patients with anterior circulation LVO stroke, the ANGEL-TNK trial will inform whether adjunct IA tenecteplase administered after successful thrombectomy reperfusion improves patient outcomes.
Trial registration number NCT05624190.
WHAT IS ALREADY KNOWN ON THIS TOPIC
A phase IIB trial showed improved excellent outcomes with intra-arterial (IA) alteplase administered after successful mechanical thrombectomy. However, this study ended early.
WHAT THIS STUDY ADDS
This study aims to evaluate whether IA tenecteplase administered after successful mechanical thrombectomy may confer improved outcomes in patients with large vessel occlusion. This protocol reviews the rationale and design of the ANGEL-TNK trial prior to data analysis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
ANGEL-TNK will inform whether the administration of tenecteplase by IA after successful reperfusion by mechanical thrombectomy may improve patient outcomes.
Background
Despite successful recanalisation after endovascular therapy (EVT) for large vessel occlusion (LVO) stroke, up to one-half of patients are dependent or dead.1–3 A discordance in successful recanalisation and clinical outcomes may be related to several factors, including evolution of cerebral infarction by the time of recanalisation, patient comorbidities or medical complications after stroke.4 A prolonged period of ischaemia can lead to changes in the cerebral microvasculature, such that even after recanalisation of an occluded vessel, there remains impairment in flow to the microvascular unit. Intraluminal platelets and fibrin thrombi may, in part, account for microvascular obstruction. The absence of cerebral microvascular reperfusion in the presence of macrovascular reperfusion is known as the ‘no-reflow’ phenomenon, and is estimated to be present in approximately one-third of patients who had a stroke with successful angiographic recanalisation of a previously occluded artery.5
The Chemical Optimization of Cerebral Embolectomy (CHOICE) trial was a phase IIb randomised trial of patients who achieved successful reperfusion (eTICI 2b50 or greater) after EVT. Patients randomised to intra-arterial (IA) alteplase after EVT had a higher likelihood of 3-month excellent functional outcomes compared with those who received placebo (modified Rankin Scale, mRS 0–1: 59% vs 40%, adjusted risk difference 18.4%, 95% CI 0.3 to 36.4%).6 However, the CHOICE trial was terminated early because of lack of placebo supply.6
The present trial aimed to evaluate whether rhTNK-tPA (recombinant human TNK tissue-type plasminogen activator) by IA thrombolysis could lead to better clinical outcomes of LVO patients after successful reperfusion after EVT between 4.5 and 24 hours from onset.
Methods
Hypothesis
The hypothesis of the ANGEL-TNK trial is that IA tenecteplase administered after a successful mechanical thrombectomy can improve 90-day excellent outcomes as compared with standard medical care.
Design
The IA rhTNK-tPA thrombolysis for acute LVO after successful mechanical thrombectomy recanalisation (ANGEL-TNK) is an investigator-initiated phase III multicentre, randomised, open-label, blinded end-point trial. The rhTNK-tPA formulation used in this trial had the same terminal amino acid sequence and different production process to the TNK made by Boehringer (Metalyse) and Genentech (TNKase), and is the same tenecteplase as that used in the TRACE, TRACE II and TRACE III trials.7–9 The optimal dose of tenecteplase for IA thrombolysis is unknown. Therefore, the steering committee collected expert opinions and recommended that the selected dose of tenecteplase for IA thrombolysis as half of that of the intravenous dose, or 0.125 mg/kg.9 10 Patients are recruited at 18 centres across China (table 1). The list of participating centres is available in online supplemental table 1.
Supplementary data
List of participating sites and investigators in ANGEL-TNK
Inclusion criteria
Clinical inclusion criteria are as follows: age >18 years; time from onset (defined as ‘last known well’, LKW) to randomization 4.5–24 hours; premorbid mRS 0–1; National Institute of Health Stroke Scale (NIHSS) >2 and the informed consent was signed by patients or their healthcare proxy.
Inclusion criteria for imaging: middle cerebral artery (MCA) M1 segment occlusion and dominant M2 segment occlusion, internal carotid artery (ICA) occlusion assessed by CT angiography (CTA) or magnetic resonance angiography (MRA); perfusion-weighted imaging (PWI) or CT perfusion (CTP) shows ischaemic infarct core volume less than 70 mL, mismatch volume no less than 10 mL and mismatch ratio of no less than 1.2; Alberta Stroke Programme Early CT Score no less than 6 assessed by diffusion-weighted imaging (DWI) or non-contrast CT (NCCT); at the end of the procedure, successful reperfusion (eTICI no less than 2b50) after EVT. Prior to EVT, patients with successful reperfusion assessed by digital subtraction angiography are also eligible.
Exclusion criteria
The main exclusion criteria include the following: use of intravenous thrombolysis (IVT) on admission (Chinese guidelines on the management of stroke does not extend the time window of IVT from 4.5 hours to 9 hours)11; contraindication to administration of thrombolysis; use of balloon angioplasty, permanent stenting and other situations during the endovascular procedure that requires antiplatelet therapy or anticoagulation within the first 24 hours; intravenous heparin administration (however, heparinised saline flushes are allowed); patients with pregnant, or positive beta-hCG test; brain tumour (with mass effect); recognised haemorrhagic diathesis, either acquired or inherited, lack of coagulation factors; known coagulopathy, international normalised ratio more than 2.0 or use of novel anticoagulant <48 hours from symptom onset; platelets less than 50×109/L; suspicion of septic emboli or endocarditis; glomerular filtration rate less than 30 mL/min or creatinine more than 2.5 mg/dL; systolic blood pressure >185 mm Hg or diastolic blood pressure more than 110 mm Hg refractory to treatment.
Exclusion criteria for imaging include midline shift or herniation, mass effect with effacement of the ventricles, evidence of acute intracranial haemorrhage on CT or MRI, acute bilateral strokes or multiple intracranial vessel occlusions.
Randomisation
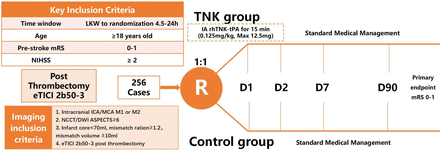
After successful reperfusion, defined as eTICI 2b50 to 3, participants are eligible to be randomised in a 1:1 ratio to receive rhTNK-tPA 0.125 mg/kg (maximum 12.5 mg) by IA+ best medical management or BMM. Eligible patients are randomly assigned according to the block method to ensure balance between and within groups. Randomisation is stratified by centre and occlusion site (ICA/MCA) (figure 1).
Study design: randomisation algorithm. ASPECTS, Alberta Stroke Programme Early CT Score; ICA, internal carotid artery; LKW, last time to know well; MCA, middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale.
Intervention
In all patients, eTICI 3 reperfusion is the primary goal with standard EVT technique. All devices used in the trial should be those approved by the National Medical Products Administration and should be performed according to their instructions for use. For patients randomised to the study arm, recanalisation of eTICI 2b50 to 3 should be confirmed before administration of rhTNK-tPA.
The administration of rhTNK-tPA 0.125 mg/kg with maximum dose 12.5 mg, will be infused slowly and constantly over 15 min according to the CHOICE trial.6 It is recommended to inject rhTNK-tPA through a microcatheter in the horizontal segment of MCA distal to the origin of the lenticulostriate branches. Penetration or passing of the clot is not allowed to avoid mechanical disruption of the clot. Anteroposterior and lateral angiography will be performed 10 min later at the end of IA infusion to record the final eTICI grading. Additionally, anteroposterior and lateral angiography will also be performed 10 min after randomisation to record the final eTICI grading in the control arm.
Safety monitoring
ANGEL-TNK has an independent Data and Safety Monitoring Board (DSMB), whose role is to monitor the safety of patients enrolled in the trial. The DSMB includes a neurologist, a neurointerventionalist and a statistician. The DSMB reviewed the interim analysis results when half of the total sample size was completed and finished the 90-day follow-up. The DSMB was blinded to patient assignment allocation. No safety concerns were identified at this time, and the trial was allowed to proceed.
Sample size
The sample size was calculated based on the phase IIb CHOICE randomised trial.5 We assumed a 40% mRS 0–1 rate in the BMM group of patients with LVO and a 19% improvement in the IA TNK group with 80% statistical power, a 5% two-sided type I error and 5% loss of follow-up. Therefore, 256 patients were required for the sample size, with 128 patients in each group. An interim analysis was conducted when the 90-day follow-up of half of the total sample size was completed, with corresponding significance levels based on O’Brien and Fleming boundary are two-sided 0.003 (interim analysis) and 0.049 (final analysis).
Efficacy endpoints
The primary endpoint is 90 (±7) days of excellent outcome defined as mRS 0–1. The secondary endpoints of the ANGEL-TNK trial include: Tmax >6 s at 24 (±12) hours; infarct core volume change from baseline, at 7 (±1) days or at discharge assessed with NCCT; 90 (±7) days mRS (shift analysis); 90 (±7) day mRS 0–2; 90 (±7) days mRS 0–3; 36 (±12) hours NIHSS 0–1 or a reduction of ≥10 from baseline NIHSS; 90 (±7) days EQ-5D-5L score. Follow-up evaluations are performed in-person or via telephone if in-person visit at the investigational site is impractical. All subjects entered into the study had a standard neurological assessment by experienced physicians who were blinded to treatment assignment.
Safety endpoints
The primary safety endpoints include symptomatic intracranial haemorrhage within 48 hours from randomisation according to the Heidelberg Bleeding Classification.12 Secondary safety endpoints include: all-cause mortality at 90 days, any intracranial haemorrhage within 48 hours from randomisation according to the Heidelberg Bleeding Classification.
Study assessments
Study assessments will be obtained on the day of randomisation, 24 (±12) hours, 7 (±1) days/discharge, 90 (±7) days. Imaging follow-up will be obtained at 24 (±12) hours assessed with NCCT+CTA+CTP or DWI+MRA+PWI as well as at 7 (±1) days or at discharge assessed with NCCT.
Statistical analysis
The main analyses for the primary and secondary outcomes will be analysed according to the intention-to-treat principle. Sensitivity analyses will be conducted in the per-protocol population. We used Wilcoxon rank-sum test to compare continuous variables and χ2 tests or Fisher’s exact test to compare categorical variables. For the primary outcome, a generalised linear model with log link and binomial error distribution with adjustment of study site and occlusion site will be used to calculate the relative risk between the two treatment groups. All statistics will be two-sided with p<0.049 considered significant.
Subgroup analysis
Additional analyses will be performed in the following subgroups: age (18–65 years vs ≥66 years), LKW to randomization time (4.5–6 hours vs 6–24 hours), baseline angiographic eTICI (eTICI 2b vs eTICI 2c/3), stroke severity before randomisation (NIHSS <16 vs NIHSS ≥16), occlusion site (ICA vs MCA), stroke type (cardiac embolism vs large-artery atherosclerosis) and admission serum glucose level (<100 mg/dL vs ≥100 mg/dL).
Study organisation
A steering committee advised the principal investigators on study conduct prior to the beginning of the trial, and during the course of the trial. The steering committee included vascular neurologists and neurointerventionalists who had experience in conducting clinical trials.
Discussion
Up to half of LVO patients achieving successful reperfusion with EVT have an unfavourable outcome.1–3 Post-reperfusion therapy may be a target of interest to further improve patient outcomes.13 The phase IIB CHOICE trial indicated that targeting the microcirculation with adjunctive IA thrombolysis to narrow the gap in angiographic and clinical outcomes may be a promising target for patients with LVO stroke.6 This is now being studied as a phase III CHOICE 2 trial (NCT05797792), with the use of IA alteplase after successful EVT (mTICI 2b/3) and the primary endpoint of microvascular hypoperfusion on 36±24-hour follow-up CTP studies. The ANGEL-TNK trial aims to investigate the benefit of adjunct IA tenecteplase administered after successful EVT in patients with anterior circulation LVO.
There are several mechanisms which can explain a discordance in clinical versus angiographic outcomes in patients who have suffered an obstruction to an artery with subsequent reperfusion. A thrombo-inflammatory response with progressive microvascular failure may occur with the influx of neutrophils, microvascular pericyte constriction and vascular remodelling with inflammation.14 In-situ or downstream microvascular thrombosis may impair perfusion at the capillary level. The concept of macrovascular reperfusion (as visible on cerebral angiography) without microvascular reperfusion (as seen on perfusion imaging) is termed the ‘no-reflow’ phenomenon, a notion described over 50 years ago.5 15
In the ANGEL-TNK trial, tenecteplase was chosen rather than alteplase because of its high fibrin specificity, safety profile and data showing improved recanalisation rates compared with alteplase in the EXTEND IA TNK trial.10 16 Moreover, randomised trials have demonstrated the non-inferiority of tenecteplase compared with alteplase in patients with ischaemic stroke.8 17 18
There are other ongoing trials that are evaluating the administration of IA tenecteplase after successful thrombectomy.19 20 The ATTENTION IA trial, which completed enrolment, examined the use of 0.0625 mg/kg of IA tenecteplase administered after successful thrombectomy in patients with posterior circulation arterial occlusion involving basilar artery, posterior cerebral artery-P1 segment or vertebral artery.18 In contrast to ANGEL-TNK, ATTENTION IA permitted inclusion of patients who received preceding IVT prior to thrombectomy, as well as angioplasty or intracranial stenting as part of the thrombectomy due to the common occurrence of intracranial atherosclerotic disease LVO in the posterior circulation. We chose not to include these patients in ANGEL-TNK for concern of haemorrhagic risk with additive antithrombotics (eg, obligate antiplatelet therapy with acute stenting to prevent stent occlusion) and to optimise the safety profile of the trial.
There are limitations of this trial. First, the treatment effect size in the CHOICE trial was large, with an absolute risk difference of approximately 19%. As our sample size calculation was based on the CHOICE data, it is possible that our trial may be underpowered to detect a statistical difference, incurring a type II error. Second, after successful EVT, it may be challenging to identify patients with early neurological improvement which would be predictive of later excellent outcomes. Inclusion of these patients in ANGEL-TNK may dilute the treatment effect of the interventional versus control arm. Future imaging techniques such as flat-panel perfusion may better discriminate patients with persistent perfusion deficits and hence select better for patients potentially candidate for IA adjunct thrombolysis.21
ANGEL-TNK enrolled its first patient on 16 February, 2022 and as of 23 March 2024, finished enrolment. Study follow-up completion is expected by June 2024.
Conclusion
The ANGEL-TNK trial will inform the safety and efficacy of adjunct IA tenecteplase administered in anterior circulation LVO patients who had a stroke who achieved successful reperfusion by EVT.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and all protocol modifications were submitted and approved by ethics committee at Beijing Tiantan Hospital (IRB approval number: KY2022-170-02) and all participating centres. The study is conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. All patients or their legal representatives must provide written consent.
Acknowledgments
We thank all the participants in the ANGEL-TNK programme for their hard work.
Footnotes
X @NguyenThanhMD, @abdalkadermd
XH, GL and DS contributed equally.
Contributors ZM, BY and XH designed the study; DS, XH and GL drafted the manuscript; Other authors provided critical comments/revisions of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.