What is already known
Recently published summaries and meta-analyses of four randomised controlled trials (RCTs)1–5 comparing direct mechanical thrombectomy (dMT) and bridging therapy with intravenous (IV) thrombolytics (alteplase) suggested that dMT is non-inferior to bridging therapy to achieve good functional outcome 3 months after stroke (modified Rankin Score 0–2) with the non-inferiority margin (NIM) <−5%.5–7 However, there were considerable limitations in generalisability as three of the RCTs were performed in the Asian population, and the alteplase dose was different between studies (0.6 mg/kg or 0.9 mg/kg). Recently, preliminary results of two further RCTs (SWIFT-DIRECT8 and DIRECT-SAFE9) were presented at the 2021 World Stroke Congress and other conferences. Both RCTs (SWIFT-DIRECT and DIRECT-SAFE) compared dMT with bridging therapy, assuming a NIM of 12% and 10%, respectively. Both failed to confirm the non-inferiority of dMT approach, although it is worth noting that DIRECT-SAFE was terminated early in June 2021, with only 293 out of planned 780 participants recruited, following the publications of the other RCTs’ results.9
What is new
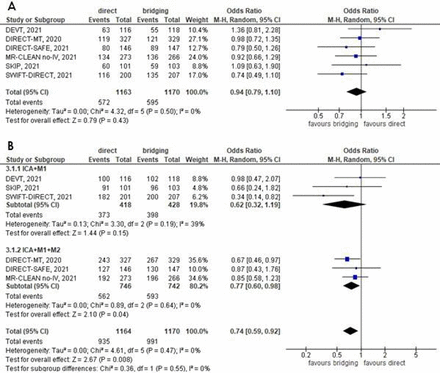
We sought to update the meta-analysis of accumulated trial data to assess the difference and non-inferiority in clinical and procedural outcomes between dMT and bridging therapy, using a random-effects model. Six RCTs comprising 2333 participants (1170 bridging therapy and 1163 dMT) were included. The non-inferiority of dMT to achieve good functional outcomes at 3 months was demonstrated with an absolute risk difference of −0.02 (95% CI −0.06 to 0.02), p=0.42, I2=0%. The lower 95% CI bound of −6% fell within the lead NIM of −10%, the strictest NIM of the included RCTs. Successful reperfusion rates (thrombolysis in cerebral infarction (TICI) ≥2b) were achieved in significantly fewer participants in the dMT group (OR=0.74 (95% CI 0.59 to 0.92), p=0.006, I2=0%), which was more frequently observed in studies that included M2 occlusions (figure 1). There was no significant difference between groups in the safety endpoints, namely mortality at 3 months (OR=1.07 (95% CI 0.85 to 1.34), p=0.56, I2=0%) and symptomatic intracranial haemorrhage (OR=0.78 (95% CI 0.51 to 1.19), p=0.25, I2=17%).
Forest plots of (A) good functional outcome, modified Rankin score 0–2, and (B) successful reperfusion (thrombolysis in cerebral infarction (TICI) 2b–3) according to the clot location.
What needs to be considered
There are ongoing concerns regarding the risks of pretreatment with IV thrombolytics in bridging therapy, including potential procedural delays, clot fragmentation and distal clot migration precluding eligibility for MT, and haemorrhagic complications. However, bridging IV thrombolysis may lyse distal thrombi, favourably alter clot properties to facilitate retrieval, leading to higher first-pass effect and successful reperfusion rates. There are also direct and indirect cost implications that need to be factored in. A recent health economic evaluation supported the economic superiority of the dMT approach based on information from the DIRECT-MT trial.10 Assuming a minimal cost of alteplase of $1, bridging therapy resulted in an additional lifetime cost of $5664/$4804 (from a healthcare and societal perspective, respectively) and a decrease of 0.25 quality-adjusted life years compared with dMT.10 This supports the need to limit the use of alteplase when it is not required, especially in low-income countries.
Limitations of the current updated analysis include the inherent risk of bias in the unpublished, non-peer-reviewed results of two of the included RCTs. Second, we lack detailed analysis of baseline characteristics that may influence the outcomes, such as the onset to revascularisation time, alteplase to groin puncture time or the proportion of participants presenting directly to MT-capable centres or those requiring secondary transfer in the ‘drip-and-ship’ model. A recent meta-analysis showed that patients admitted directly to MT-capable centres had higher odds of achieving good functional outcome (OR=1.26 (95% CI 1.12 to 1.42); p<0.001) compared with those in the ‘drip-and-ship’ model, although there were no differences in outcomes in the subgroup of patients who underwent bridging therapy.11 Additionally, the observational study by Purrucker et al suggested that initiation of the thrombolysis prior to the transfer between primary and comprehensive stroke centres is associated with increased odds of early recanalisation (OR=10.9 (95% CI 3.8 to 31.1); p<0.001).12 This aspect is of utmost importance, as it may be reasonable not to withhold IV treatment securing the patient during the long transport time. Last, only alteplase was used in the bridging therapy group, precluding comparisons of alternative thrombolytics, such as tenecteplase, which has proven to be associated with greater odds of successful reperfusion and early neurological improvement without the increase in the incidence of safety outcome.13
In summary, combined trial data showed dMT is non-inferior to bridging therapy in achieving good functional outcomes at 3 months with a 6% margin of confidence in patients presenting directly to centres providing dMT (based on available data). An independent patient data meta-analysis should clarify the validity of these findings across different subgroups and under-represented patient populations in each trial.
Ethics statements
Patient consent for publication
Ethics approval
This study does not involve human participants.
Footnotes
Contributors Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work—AP, PSD, WB, IQG and TJE. Drafting the work or revising it critically for important intellectual content—AP, PSD, WB, IQG and TJE. Final approval of the version to be published—AP, PSD, WB, IQG and TJE. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved—AP, PSD, WB, IQG and TJE.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.