Abstract
As intracerebral hemorrahge becomes more frequent as a result of an aging population with greater comorbidities, rapid identification and reversal of precipitators becomes increasingly paramount. The aformentioned population will ever more likely be on some form of anticoagulant therapy. Understanding the mechanisms of these agents and means by which to reverse them early on is critical in managing the acute intracerebral hemorrhage.
Introduction
Intracerebral haemorrhage (ICH) is a disease entity associated with high morbidity and mortality. It accounts for nearly 15% of all strokes.1 In the USA, the mortality rate of patients with ICH is nearly 34% confirming the increasing burden of the disease.2–4 Oral anticoagulation is a common cause of ICH and the use of oral anticoagulation is estimated to continually rise given increasing rate of atrial fibrillation detection.5 Coagulopathy-associated ICH results in poor functional outcomes given rapid haematoma expansion as early as 1 hour.1–3
This article will review the indications, efficacy and safety of vitamin K antagonists (VKAs) as well as direct-acting oral anticoagulants (DOACs) and outline the reversal of coagulopathy by these agents in ICH.
Comparison of efficacy and safety between DOACs and VKAs
Direct thrombin inhibitors and factor Xa inhibitors (FXa-Is) constitute the two classes of DOACs (table 1). Dabigatran (Pradaxa, Boehringer Ingelheim Pharmaceuticals) is a direct thrombin inhibitor, whereas rivaroxaban (Xarelto, Janssen Pharmaceuticals), apixaban (Eliquis, Bristol-Myers Squibb Company), edoxaban (Savaysa and Lixiana, Daiichi Sankyo) and betrixaban (Bevyxxa, Portola Pharmaceuticals) fall in the category of FXa-Is. DOACs do not require frequent monitoring of the international normalised ratio (INR) and have shorter half-lives with fewer drug interactions, making them more favourable for use than warfarin.6 Recent trials including ARISTOTLE (apixaban),7 8 RE-LY (dabigatran),9 ROCKET AF (rivaroxaban),10 ENGAGE AF-TIMI 48 (edoxaban)10 on stroke and systemic embolism have shown non-inferiority of DOACs when compared with warfarin. The rate of bleeding (including major haemorrhage, fatal haemorrhage, haemorrhagic stroke or ICH) is also lower for DOACs at 3% to 4% when compared with warfarin at 5% to 6%. Additionally, the rate of only ICH is lower for DOACs at 0.3% to 0.4% when compared with warfarin at 0.7% to 0.8%.7–11
Direct-acting oral anticoagulants (DOACs) and reversal
The rate of ischaemic stroke in patients taking DOACs has been noted to be higher when compared with warfarin.12–14 This could be explained by inconsistent use of DOACs resulting in subtherapeutic levels given their short half-lives.15
The outcome of ICH while on DOACs remains a topic for research. CROMIS-2 (The Clinical Relevance of Microbleeds in Stroke Study) compared all-cause 90-day mortality, functional outcome, ICH volume and haematoma expansion between patients with ICH associated with VKA and ICH associated with DOAC. There were no significant differences between the two groups.16 Other studies have shown similar functional outcomes in patients with ICH receiving VKA and DOACs and also mortality benefit in patients on DOACs.17 18
DOACs are becoming the preferred agents for oral anticoagulation when compared with VKAs given their safety profile.19 There is limited data on the reversal protocol of DOACs. As their use will continue to rise, it is imperative to understand the management of DOAC related ICH.
Reversal of VKA-related coagulopathy
Pharmacology of VKAs
Warfarin interferes with production of vitamin K dependent clotting factors II, VII, IX, X by depleting vitamin K reserve.20 Warfarin is metabolised by cytochrome P450 enzyme, which can be inhibited or induced by a variety of drugs resulting in variable metabolism of warfarin.
Reversal
Vitamin K is available in oral, subcutaneous and intravenous preparations for patients with life threatening bleeding. Intravenous vitamin K is most efficacious among the three with a recommended dose of 10 mg intravenously.21 However, INR normalisation with vitamin K can take up to a day1–3 22 and therefore it is not sufficient alone in the management of ICH. It is usually given in combination with fresh frozen plasma (FFP) or prothrombin complex concentrate (PCC) (table 2).
Anticoagulant reversal agents and their pharmacokinetics
FFP is the liquid portion derived from whole blood. It corrects coagulopathy by replacing plasma proteins to replete clotting factors. FFP reversal of INR can take up to 30 hours making it an ineffective treatment of early haematoma expansion.23–25 It requires high volumes and can worsen fluid balance in patients with heart failure resulting in pulmonary oedema as well as transfusion reactions.26
PCC is comprised of clotting factors II, IX and X at levels significantly higher than FFP. Activated PCC (aPCC) also contains factor VII in addition to II, IX and X. PCC results in rapid INR reversal and is not associated with complications such as fluid overload as seen with FPP.27 The INCH trial (International Normalised Ratio Normalisation in Coumadin-Associated Intracerebral Haemorrhage) compared 30 IU/kg dose of PCC to 20 mL/kg FFP in reversal of VKA associated ICH.25 PCC had a higher rate of INR reversal to <1.3 within 3 hours when compared with FFP (67% vs 9%; p=0.0003). The average time of INR reversal was 40 min in PCC group when compared with >24 hours for FFP and haematoma expansion was less for PCC group at 3 and 24 hours.28 The rate of thrombotic complications remains similar between PCC and FFP.29–32 PCC (more than US$5000 for 20 IU/kg dose in a 70 kg patient) is more expensive when compared with FFP (less than US$2000 per unit), however it is more cost-effective given its advantages over FFP as outlined above. aPCC (20 IU/kg) is also found to be more efficacious than FFP without significant increase in thrombotic complications.33 There are no studies comparing aPCC to PCC to date.
Reversal of DOAC-related coagulopathy
Pharmacology of DOACs
Dabigatran directly competes with thrombin, thus inhibiting production of fibrin. It has a half-life of approximately 15 hours and is primarily cleared by the kidneys.34 FXa-Is act by binding and inhibiting factor Xa with half-lives of 6 to 12 hours, except for betrixaban which is approximately 24 hours.35 They are also primarily cleared by the kidneys.36 Unfortunately, unlike warfarin, dabigatran has no modality, which accurately measures its anticoagulation effect. Currently, specific anti-Xa assays are available for the FXa-Is but they are not widely available, have a complex measurement system and are relatively expensive (over US$20 per test).37
Reversal
In vitro studies have demonstrated PCC efficacy in reversal of DOAC anticoagulation.38–41 Ex vivo studies have been done on small populations of healthy male volunteers that proved the efficacy of both three factor PCC and four factor PCC at 50 IU/kg. Both types of PCC decreased PT within 30 min.42–44 Multiple trials have been conducted in actively bleeding patients (table 3). The UPRATE trial (Unactivated Prothrombin Complex Concentrates for the Reversal of Anti-Factor Ten Inhibitors) showed that four factor PCC reliably reversed 84 patients with major bleeding on apixaban and rivaroxaban (70% with ICH, 15% with gastrointestinal bleed). The overall mortality was 32% at 30-day follow-up for both drugs.45–47
Summary of trials for anticoagulant reversal and haemorrhage treatment
There are anticoagulant reversal agents specific for different DOACs (tables 1 and 2). Idarucizumab is a monoclonal antibody that binds dabigatran. REVERSE-AD trial (Reversal Effects of Idarucizumab on Active Dabigatran) enrolled patients with active bleeding and patients that need reversal of anticoagulation prior to a procedure. Unfortunately, the study was was not designed for patients with ICH. Therefore it lacked objective measurement of clinical haemostasis in ICH.46 Nevertheless, idarucizumab was shown to reverse the effect of dabigatran for approximately 93% of patients within minutes.48
Recently, the Food and Drug Administration approved andexanet alfa, the second DOAC specific reversal agent. It is an elegant reversal agent for FXa-Is. It is a recombinant FXa that binds FXa-I. The ANNEXA-4 trial (Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of FXA Inhibitors) had 168 patients with ICH.47 Haemostatic efficacy was measured via serial CT scans. Of those with ICH, 80% achieved excellent or good haemostasis 12 hours after infusion. Excellent haemostasis was defined as haematoma growth <20% and good as <35% at 12 hours. The study has limitations, it excluded patients with ICH with Glasgow Coma Scale <7 and ICH with volume >60 mL.47
On recombinant activated coagulation factor VII
There is a growing interest in recombinant activated factor VII (rFVIIa) in all forms of active bleeding associated with coagulopathy. It was recently compared with placebo in patients with ICH and a spot sign on CT angiography. The study analysed 69 patients pooled from the SPOTLIGHT (‘Spot Sign’ Selection of Intracerebral Haemorrhage to Guide Haemostatic Therapy) and STOP-IT (The Spot Sign for Predicting and Treating ICH Growth Study) trials. These trials were closely coordinated between the investigators in Canada (SPOTLIGHT) and the USA (STOP-IT). Patients were given rFVIIa within 3 hours of stroke onset. The primary outcome was parenchymal ICH volume expansion and the secondary outcome was total haemorrhage volume expansion on head CT at 24 hours. The drug did not improve radiographic findings or outcomes compared with placebo. Baseline media ICH volumes were 19.6 (9.6 to 39.2) mL in the rFVIIa group and 20.4 (8.6 to 32.6) mL in the placebo.49 This further adds to the growing evidence against rFVIIa as a reversal agent.
Conclusion
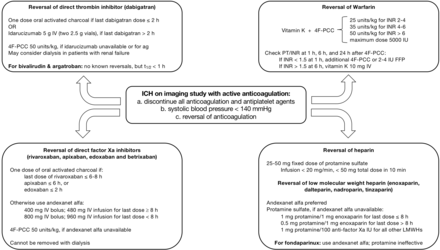
Anticoagulation associated with ICH remains under investigated. Ironically, an indication for anticoagulation is prevention of stroke. Though studies are showing DOACs lower the risk of bleeding associated complications compared with VKAs, there is paucity of strong data for the reversal of coagulopathy in ICH. Larger and more robust clinical trials targeting ICH are needed. Reversal agents need direct comparison against four factor PCC. Based on the current literature and standard of care, we developed a suggested algorithm for coagulopathy reversal in ICH (figure 1).
Algorithm for the reversal of specific anticoagulants, including direct thrombin inhibitors, direct factor Xa inhibitors, warfarin and heparin. FFP, fresh frozen plasma; 4F-PCC,four factor prothrombin complex concentrate;ICH, intracerebral haemorrhage; INR, internationalnormalised ratio; IU, international unit; IV,intravenous; LMWH, low-molecular weight heparin; PCC,prothrombin complex concentrate.
Footnotes
Twitter @neuro_md
Contributors AS and NS contributed to drafting, revising and finalising the manuscript. JC revised the manuscript critically for important intellectual content and prepared tables. MB, LG and MS revised critically for important intellectual content. WY revised critically for important intellectual content and finalised the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Commissioned; externally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.