Abstract
Stroke is a cerebrovascular disease displaying high mortality and morbidity. Despite extensive efforts, only very few therapies are available for stroke patients as yet. Hydrogen sulfide (H2S) is thought to be a signalling molecule that is endogenously produced and plays functional roles in the central nervous system. Currently, numerous studies show that H2S impacts stroke outcomes in animal and cellular models. Here, we review the recent research regarding the effects of endogenously produced H2S as well as exogenous H2S donors on stroke pathology, focusing on the potential of H2S-based therapies in treating ischaemic stroke. We also discuss the several issues that hinder the clinical translation of H2S-based therapies from the bench. Taken together, we think that H2S-based therapies are promising strategies for treating cerebral ischaemia if we successfully address these issues.
Introduction
Stroke is the second leading cause of death and leading cause of adult disability worldwide, with cerebral ischaemia as the major form of stroke, which approximately accounts for more than 85% of all strokes.1 Currently, the therapies for ischaemic stroke are rather limited, with tissue plasminogen activator (tPA) as the only drug treatment approved by the US Food and Drug Administration for acute ischaemic stroke. Promising evidence from the clinical trials is emerging to suggest that patients with stroke can benefit from endovascular management strategies due to the restoration of cerebral blood flow. However, the recovery of cerebral blood flow, theoretically, cannot rescue the brains cells in the rim of infarct cores, which rapidly undergo death triggered by ischaemic cascades. Thus, the development of additive treatments that either provide acute brain protection or promote long-term functional recovery is of clinical significance to improve the beneficial effects of endovascular reperfusion strategies.
Hydrogen sulphide (H2S) is traditionally considered a toxic gas. However, the research of the past two decades has revealed that H2S is a gasotransmitter that displays profound and pleiotropic effects both physiologically and pathologically.2 Particularly, large amount of evidence suggests that H2S, as a gasotransmitter, plays important functional roles in the central nervous system.3 4 The neuroprotective abilities of H2S have been investigated in the context of ischaemic stroke. Here, we briefly review the newest reports about the effects of H2S on the outcomes following cerebral ischaemia as well as underlying mechanisms. We also discuss the important issues that challenge the clinical translation of H2S-based therapies.
Physiological functions of H2S and its roles in ischaemic stroke
The role of endogenously produced H2S in ischaemic stroke
Few studies have been devoted to investigate the role of endogenously produced H2S, the level of which is markedly increased after middle cerebral artery occlusion (MCAO),5 6 in the pathology of ischaemic stroke. Currently available publications suggest the endogenously produced H2S contributes to stroke pathology.
H2S is endogenously synthesised by three enzymes, namely cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS) and 3-mercaptopyruvate sulfurtransferase (3MST) in conjunction with cysteine aminotransferase.7 CSE is in abundance in the cardiovascular system and produces H2S from cysteine.8 CSE expression is minor in the brain, and consistently, CSE inhibitors do not suppress the production of H2S in the rat brain.3 8 CBS is considered the predominant H2S synthesising enzyme in the brain. CBS is mainly expressed by astrocytes, and could also be expressed by microglia and neurons in the brain.9 β-replacement of cysteine with homocysteine to H2S and cystathionine, the most kinetically efficient reaction catalysed by CBS, contributes up to 95% of the net production of H2S by CBS in the brain.10 It has been shown that cysteine administration increases the infarct damage dose-dependently, which is abolished by the CBS inhibitor aminooxyacetic acid (AOAA).5 This indicates that endogenously produced H2S from cysteine is likely involved in ischaemic brain damage. Indeed, high plasma cysteine levels within 24 hours of stroke onset have been shown to be positively correlated with early stroke deterioration and long-term clinical outcomes assessed at 3 months.11 Wong’s group shows that H2S production via 3MST is reduced in astrocytes after stroke, further suggesting that CBS is responsible for the elevation of H2S level following cerebral ischaemia.12 Moreover, by using a permanent MCAO, Wong’s group showed that MCAO enhances both H2S levels and H2S synthesising activities in the ischaemic cortex. Besides, infarct damage is reduced by four inhibitors of H2S synthesis enzymes.5
However, a recent study found that AOAA confers protective effects at low dose, although at higher doses it does not protect or even worsen the ischaemic injury after MCAO.13 Moreover, our group showed that CBS expression and H2S synthesising activity were significantly reduced in the microglia in the ischaemic brain.14 Moreover, we showed that overexpressing CBS in microglia in vitro or supplementing the exogenous H2S donor in vivo suppressed microglia-mediated neuroinflammation and reduced infarct damage in the ischaemic models.14 Collectively, these results suggest that endogenously produced H2S in microglia via CBS plays a protective role by inhibiting neuroinflammation following cerebral ischaemia. Thereby, more research is needed to shed light on the role of endogenous H2S in stroke pathology.
The effects of exogenous H2S donors on stroke outcomes following cerebral ischaemia
Conflicting evidence exists for the effects of H2S donors on stroke outcomes following cerebral ischaemia. The discrepancy may result from the use of different doses of inorganic H2S donors such as sodium hydrogen sulfide (NaHS) in the previous studies.
The first study about the effects of H2S on stroke outcomes was published in 2006. The study showed that NaHS at 180 mmol/kg, but not at 90 mmol/kg (intraperitoneal injection), exacerbated infarct damage following MCAO in rats.5 Consistent with the publication, a paper published later also reported that NaHS at 180 mmol/kg remarkably exacerbated neuronal damage at 7 days of reperfusion in a rat global ischaemia-reperfusion model induced by permanent bilateral occlusion of vertebral artery and followed by 15 min of occlusion of common carotid artery, and no effects were detected at 90 mmol/kg.6 The deleterious effects of H2S following cerebral ischaemia are likely to be mediated by two mechanisms. First, H2S is a potent inhibitor of cytochrome c oxidase (complex IV) comparable to cyanide.15 The inhibition is reversible with a Ki of 10–30 µM for isolated mitochondria or cultured cells.16 H2S donors, especially inorganic H2S donors that release excessive H2S instantaneously, are expected to impair mitochondrial functions adversely at concentrations used in the previous studies. It is likely that toxic inhibition on complex IV mainly contributes to the deleterious effects observed with inorganic H2S donors following ischaemic stroke. Moreover, H2S has been shown to activate N-methyl-D-aspartate (NMDA) receptor function following ischaemic stroke.17 Indeed, NMDA receptor antagonists inhibit H2S-induced cell death in neurons18 and reduce infarction in vivo,5 suggesting that H2S exacerbates infarct injuries by activating NMDA receptors.
Current research suggests a dose-response pattern for the effects of H2S donors on stroke outcomes following cerebral ischaemia.19 Strikingly, it is found that NaHS at 25 mmol/kg displayed remarkable neuroprotection against cerebral ischaemia-reperfusion injury in the same model.6 The group also reports that NaHS at the same low dose significantly lowers mortality, improves neurological deficit and reduces infarct volume in rats following transient MCAO.20 More recently, we also report that NaHS at 25 mmol/kg decreases infarct volumes and protects against the disruption of the blood-brain barrier in the well-established mouse transient MCAO model.21 Moreover, we show that NaHS at low dose attenuates haemorrhagic transformation induced by tPA following cerebral ischaemia in the mouse transient MCAO model.22 In addition, NaHS at 5 mg/kg significantly improves functional outcomes after 2 weeks in a rat MCAO model likely by augmenting angiogenesis in the peri-infarct area,23 suggesting a potential value of H2S donors in regenerative recovery after stroke.
The slow-releasing organic H2S donors release H2S in a controlled manner. However, very few studies have investigated the effects of organic donors on stroke outcomes. The most widely used organic H2S donor is 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione (ADT-OH), which requires cellular endogenous enzymes to release H2S.24 We report that ADT-OH significantly inhibits microglia-mediated neuroinflammation upon lipopolysaccharide (LPS) stimulation.25 Consistently, by using a transient mouse MCAO model, we show that 5-(4-methoxyphenyl)-3H-1,2-dithiole-3-thione (ADT), which is metabolised into ADT-OH in vivo, reduces infarct volumes and prevents disruption of the blood-brain barrier following MCAO via inhibiting the proinflammatory nuclear factor-κB (NF-κB) axis, matrix metalloproteinases 9 (MMP9) expression and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase isoform 4-derived reactive oxygen species (ROS) production in the ischaemic brain.21 Moreover, ADT is also able to inhibit haemorrhagic transformation induced by tPA following cerebral ischaemia in the mouse transient MCAO model.22 In an innovative study by another group, the H2S donor moiety ADT-OH is combined with an NMDA antagonist (memantine) and the product is an H2S-releasing NMDA antagonist (S-memantine). Both ADT-OH and S-memantine protect against cell death in human neuroblastoma SH-SY5Y cells and primary neurons induced by oxygen and glucose deprivation. Importantly, S-memantine shows greater protective effects at the same concentration than ADT-OH does. In mice receiving bilateral carotid artery occlusion followed by reperfusion, at the dose of 25 mmol/kg, only S-memantine improves the survival rate and neurological deficits.26
Although inhalation of H2S does not find its use in clinical practice, inhalation of H2S gas has been shown to be protective in animal models following cerebral ischaemia. For instance, H2S inhalation at 40 ppm and 80 ppm decreases infarct volumes and brain oedema by suppressing the expression of aquaporin-4 (AQP-4) through activating protein kinase C in rats following MCAO.27 Consistently, preconditioning with H2S inhalation decreases cerebral ischaemia/reperfusion injury and improves cognitive impairment in mice by the induction of HSP70 through the PI3K/Akt/Nrf2 pathway.28 Inhalation of 80 ppm H2S leads to marked and reversible reduction of metabolic rate in mice,29 indicating that H2S breathing may protect organ function when the nutrients and oxygen supply are compromised, such as after ischaemic stroke and cardiac arrest. Moreover, in a rabbit cardiac arrest model, inhalation of 80 ppm H2S reduces damage histologically and improves neurological functions during the acute phase.30 More interestingly, inhalation of H2S gas has been shown to induce long-term hypothermia (2 days) and thus reduce infarct damage in aged rats after MCAO.31
Mechanisms underlying the effects of H2S in ischaemic stroke
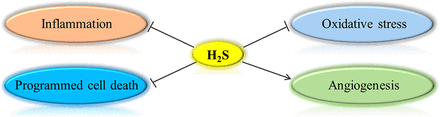
Several mechanisms may contribute to the therapeutic effects of H2S. Following cerebral ischaemia, ischaemic insults trigger inflammation, oxidative stress, apoptotic cell death and endoplasmic reticulum stress, leading to ischaemic injuries. Our group shows that activation of adenosine 5‘-monophosphate-activated protein kinase (AMPK) is a major mechanism underlying H2S inhibition on neuroinflammation.25 Particularly, we show that both slow-releasing H2S donors and endogenously produced H2S protect against cerebral ischaemia by activating AMPK to inhibit microglia-mediated neuroinflammation.14 NaHS at low doses reverses the ischaemia-induced elevation in malondialdehyde levels and decrease in Cu/Zu superoxide dismutase and glutathione (GSH) peroxidase activities in rats following transient MCAO,32 suggesting that H2S may ameliorate cerebral ischaemic injuries by suppressing postischaemic oxidative stress. In addition, nuclear translocation of apoptosis-inducing factor (AIF) and poly(ADP-ribose) polymerase-1 (PARP-1) in the ischaemic brain is attenuated by NaHS in rats following transient MCAO,32 indicating that NaHS likely reduces caspase-independent cell death by suppressing PARP-1/AIF signalling. Both NaHS at low dose and ADT protect blood-brain barrier integrity following mouse MCAO.21 Moreover, NaHS promotes angiogenesis in the peri-infarct area after ischaemic stroke, possibly through augmenting the phosphorylation of AKT and extracellular signal‐regulated kinase (ERK) and increasing the expression of angiopoietin-1 and vascular endothelial growth factor.23 Collectively, current evidence suggests that H2S confers protection against cerebral ischaemia via the mechanisms of anti-inflammation, anti-oxidative stress, anti-programmed cell death and proangiogenesis (figure 1).
Mechanisms underlying the effects of hydrogen sulfide (H2S) in ischaemic stroke.
The challenges of H2S-based therapies for ischaemic stroke
Several issues that have not been solved in the biology and effects of H2S following cerebral ischaemia hinder the clinical translation of H2S-based therapies from the bench. First, the signalling mechanisms underlying the therapeutic effects of H2S remain obscure. By definition, as a gasotransmitter, the effects of H2S should be specifically mediated by a limited number of cellular or molecular targets. Currently, the predominant H2S signalling mechanism is sulfhydration of target proteins. Since it is estimated that 10%–25% cellular proteins can be sulfhydrated, the mechanism is unspecific. Moreover, we do not know whether the therapeutic mechanisms of H2S can be separated from its toxic mechanisms, such as inhibition on complex IV. Second, we know very little regarding the role of endogenous H2S in stroke pathology. Current studies mainly use the inhibitors of H2S synthases to investigate the role of endogenous H2S in stroke pathology. Since the unspecificity of these inhibitors, no clear conclusion has been reached based on these studies. We suggest that genetically engineered mice with deletion in H2S synthases, especially mice with cell-specific deletion of H2S synthases, will be the unique and valuable tools for the investigation of the role of endogenous H2S in stroke pathology. Third, current studies almost exclusively focus on the acute protection of H2S following cerebral ischaemia. Since most of the currently available stroke therapeutic targets display ‘biphasic’ effects,33 it is important to examine the effects of H2S on long-term stroke outcomes.
Conclusion markers
Current evidence has suggested that H2S confers acute protection as well as promotes long-term functional recovery following cerebral ischaemia. Since inorganic H2S donors instantaneously release excessive H2S and high dose of inorganic H2S donors exacerbate brain injury after cerebral ischaemia, slow-releasing organic donors, especially those requiring endogenous enzymes to release H2S, open the opportunities to treat cerebral ischaemia with H2S-based therapies. Further investigations are needed to elucidate the underlying signalling mechanisms mediating the therapeutic effects of H2S, and we believe that H2S-based therapies can be translated into clinical practice if we successfully address the issues.
Footnotes
JJ and JL contributed equally.
Contributors JJ contributed to manuscript drafting; JL contributed to manuscript revision; JC reviewed and approved the final manuscript.
Funding This work was supported by the following grants: National Natural Science Foundation of China (81671310, 81571124 and 81471336), Priority Academic Program Development of the Jiangsu Higher Education Institutions, Suzhou Clinical Research Center of Neurological Disease (Szzx201503) and the Jiangsu key laboratory grant (BM2013003).
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
Data sharing statement No additional data are available.
Patient consent for publication Not required.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.