Abstract
The optimal dose of recombinant tissue plasminogen activator (rtPA) for acute ischaemic stroke (AIS) remains controversial, especially in Asian countries. We aimed to update the evidence regarding the use of low-dose versus standard-dose rtPA. We performed a systematic literature search across MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), PsycINFO and Cumulative Index to Nursing and Allied Health Literature (CINAHL) from inception to 22 August 2016 to identify all related studies. The outcomes were death or disability (defined by modified Rankin Scale 2–6), death, and symptomatic intracerebral haemorrhage (sICH). Where possible, data were pooled for meta-analysis with ORs and corresponding 95% CIs by means of random-effects or fixed-effects meta-analysis. We included 26 observational studies and 1 randomised controlled trial with a total of 23 210 patients. Variable doses of rtPA were used for thrombolysis of AIS in Asia. Meta-analysis shows that low-dose rtPA was not associated with increased risk of death or disability (OR 1.13, 95% CI 0.95 to 1.33), or death (OR 0.86, 95% CI 0.74 to 1.01), or decreased risk of sICH (OR 1.06, 95% CI 0.65 to 1.72). The results remained consistent when sensitivity analyses were performed including only low-dose and standard-dose rtPA or only Asian studies. Our review shows small difference between the outcomes or the risk profile in the studies using low-dose and/or standard-dose rtPA for AIS. Low-dose rtPA was not associated with lower risk of death or disability, death alone, or sICH.
Background
Recombinant tissue plasminogen activator (rtPA) is the established treatment for acute ischaemic stroke (AIS). Most guidelines1 2 recommend a dose of 0.9 mg/kg of rtPA (10% as bolus and the remaining as an infusion over 1 hour; maximum dose 90 mg) to eligible patients with AIS, presenting within 3 or 4.5 hours of symptom onset, based on the National Institute of Neurological Disorders and Stroke (NINDS)3 and the European Cooperative Acute Stroke Study (ECASS) trials,4–6 respectively. However, a dose of 0.6 mg/kg (10% as bolus and the remaining as infusion over 1 hour; maximum dose 60 mg) is the approved dose of rtPA in Japan,7 where non-randomised studies8–11 have shown comparable clinical outcomes and reduced risk of symptomatic intracerebral haemorrhage (sICH) compared with the standard dose.8 9 In other Asian countries, low-dose rtPA is used widely, largely due to the reduced cost and lower anticipated rates of sICH.12 13
The recently published ENhanced Control of Hypertension ANd Thrombolysis strokE stuDy (ENCHANTED)13–15 was a randomised controlled trial to determine the impact of low-dose rtPA in patients with AIS who are eligible to receive thrombolysis treatment. It demonstrated that low-dose rtPA did not meet the non-inferiority criteria compared with standard-dose with respect to the conventional binary clinical endpoint of death and disability, defined by scores of 2–6 on the modified Rankin Scale (mRS) at 90 days. However, low-dose rtPA was non-inferior with respect to an ordinal analysis of this endpoint, and there was significantly less sICH with low-dose rtPA. The results of the ENCHANTED trial raised questions about the widespread use of low-dose rtPA in Asian medical practice.
Therefore, we have updated the systematic review,12 which influenced the design on the ENCHANTED trial was, to synthesise and provide comprehensive, updated evidence on the use of low-dose rtPA in AIS.
Methods
Study selection criteria
This systematic review adhered to the guidelines of the Meta-analysis Of Observational Studies in Epidemiology.16 There were no language restrictions.
Study eligibility criteria were the same as the previous systematic review12 17 and included those that reported functional outcomes at 3 months and documented rates of sICH.
Databases and sources
A comprehensive search strategy (online supplementary table S1), developed in consultation with a university librarian, neurologists and epidemiologists, was used to address the unique features and indexing of each of the five electronic databases (MEDLINE, Embase, Central, PsycINFO and Cumulative Index to Nursing and Allied Health Literature (CINAHL)). These were searched from inception to 22 August 2016. The reference lists of all articles that met the inclusion criteria were examined to identify studies that may have been missed by the database search.
Supplementary file 1
Data collection and extraction
XW and ShY independently scrutinised the titles and abstracts, and excluded clearly irrelevant references. XW and ShY extracted data and assessed the quality of study independently from the included studies. The methodological quality of each eligible observational study was graded using the Newcastle-Ottawa Scale.18 The quality of the ENCHANTED trial was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria,19 and the risk of bias was assessed using the Cochrane Collaboration tool. A third author (DZ) was consulted for any disagreement, which was ultimately resolved by consensus.
Data analysis
We calculated the proportions of subjects in each study with death or disability (mRS 2–6), death, and sICH with their CIs using the following formula: 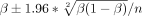 . For the meta-analysis, OR from the individual studies was pooled with the random-effects or fixed-effects methods, based on included study heterogeneity. Otherwise, a narrative review of studies was presented. The degree of heterogeneity was calculated using the I2 index. We regarded I2 of <40% as minimal, 40%–74% as modest and >74% as considerable.20
. For the meta-analysis, OR from the individual studies was pooled with the random-effects or fixed-effects methods, based on included study heterogeneity. Otherwise, a narrative review of studies was presented. The degree of heterogeneity was calculated using the I2 index. We regarded I2 of <40% as minimal, 40%–74% as modest and >74% as considerable.20
All statistical analyses were performed at a significance level of 0.05 using Stata V.11 software.
Results
Characteristics of included studies
Of 480 references obtained by our search strategy, 27 studies (23 210 patients) satisfied the eligibility criteria and were included in the final analyses (online supplementary figure S1 and table S2). It is important to note that there was only one randomised controlled trial,14 and the remaining 26 were observational studies.8–11 21–42 As none of these observational studies performed an ordinal (SHIFT) analysis of the functional outcome, we were unable to include this outcome among our analyses. Two studies were international,14 34 four each from China,28 29 33 42 Taiwan22–25 and India,21 32 35 36 seven from Japan,8–11 27 39 41 two from Thailand,30 38 and one each in South Korea,26 Pakistan,40 Singapore37 and Vietnam.31 All studies documented the mean age of the subjects (range: 53–81.7 years) and the National Institutes of Health Stroke Scale (NIHSS) score on presentation (median range: 8.7–20 points). The onset to treatment time was recorded in all the studies and ranged from a mean of 126 to 170 min. The graded quality of the included studies is listed in online supplementary table S3.
rtPA dose
As previously reported, variable doses of rtPA were used for thrombolysis of AIS in Asia. In 19 studies,8–11 14 21 25–30 32 34 36 38–41 patients were treated with either a standard-dose rtPA (0.9 mg/kg) or low-dose rtPA (0.6 mg/kg). The remaining eight studies22–24 31 33 35 37 42 employed variable rtPA dose regimens, ranging from 0.5 mg/kg to 0.9 mg/kg body weight.
Functional outcomes
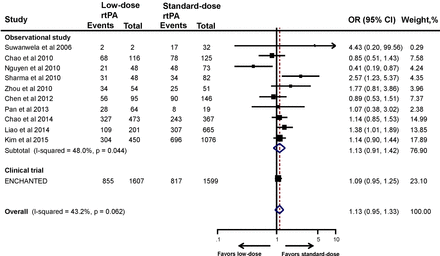
Functional outcomes include composite of death or disability (mRS 2–6) and death (mRS 6) alone. The information on functional outcome was not available in five studies.32 35 36 38 40 The proportion of patients treated with standard-dose rtPA who suffered poor 3-month outcome (mRS 2–6)14 21–23 25 28 30–34 36–38 40 42 ranged from 41.5% to 67%, which is comparable with that in the NINDS trial3 and the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST).43 The pooled estimate of 10 observational studies showed no significant association between low-dose rtPA and increased risk of death or disability (OR 1.13, 95% CI 0.91 to 1.42) (figure 1). After adding the ENCHANTED trial, the pooled estimate remained the same (OR 1.13, 95% CI 0.95 to 1.33). Sensitivity analyses of the studies that included only low-dose and standard-dose rtPA (OR 1.08, 95% CI 0.96 to 1.21) (online supplementary figure S2) and/or only Asian patients (OR 1.13, 95% CI 0.95 to 1.34) provided consistent results (online supplementary figure S3).
Association between the rtPA dose and death or disability. ENCHANTED, ENhanced Control of Hypertension ANd Thrombolysis strokE stuDy; rtPA, recombinant tissue plasminogen activator.
The proportion of patients treated with standard-dose rtPA and dying within 3 months in most studies14 21–23 25 28 30–34 36 37 40 42 varied from 1.8% to 19% and was comparable with that in the NINDS trial and in the SITS-MOST registry. Pooled estimate of nine observational studies in figure 2 showed that low-dose rtPA did not increase the risk of death (OR 0.92, 95% CI 0.74 to 1.14). This association did not change (OR 0.86, 95% CI 0.74 to 1.01) when the results from the ENCHANTED trial were combined with the observational studies (figure 2).
Association between the rtPA dose and death. ENCHANTED, ENhanced Control of Hypertension ANd Thrombolysis strokE stuDy; rtPA, recombinant tissue plasminogen activator.
Safety outcomes
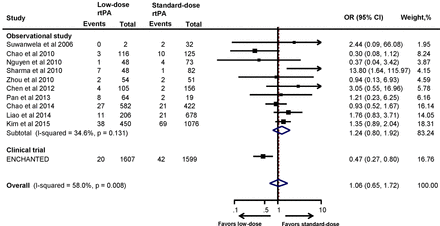
The proportion of sICH as per NINDS, SITS-MOST and ECASS criteria is summarised in online supplementary table S1. Meta-analysis of the included studies did not demonstrate any significant reduction of sICH with low-dose rtPA when compared with the standard-dose rtPA, and this observation did not change even with the pooled results (OR 1.24, 95% CI 0.80 to 1.92). This is in contrast with the results of the ENCHANTED trial, where low-dose rtPA reduced the risk of sICH significantly compared with standard-dose rtPA (OR 0.47, 95% CI 0.27 to 0.80). Interestingly, when the results of the ENCHANTED trial were combined with the observational studies, this association became non-significant (OR 1.06, 95% CI 0.65 to 1.72) (figure 3). The results remained consistent when sensitivity analyses of studies that included only low-dose and standard-dose rtPA (OR 0.92, 95% CI 0.47 to 1.80) (online supplementary figure S4) or only Asian studies (OR 1.04, 95% CI 0.63 to 1.73) were performed (online supplementary figure S5). As shown in online supplementary table S1, the proportion of sICH (NINDS criteria) with standard-dose rtPA in the Asian studies14 21–23 25 32–34 36 42 was comparable with the results of the NINDS trial.3 Similarly, studies that employed SITS-MOST definition for sICH22 23 26 27 30–32 34 37 produced results comparable with43 SITS-MOST registry.
Association between the rtPA dose and symptomatic intracranial haemorrhage. ENCHANTED, ENhanced Control of Hypertension ANd Thrombolysis strokE stuDy; rtPA, recombinant tissue plasminogen activator.
Discussion
In this systematic review, low-dose rtPA did not increase the risk of death or disability, death alone, or decreased the risk of sICH. Sensitivity analyses including studies with only low-dose and standard-dose rtPA and with only Asian patients with AIS demonstrated consistent results. The combined endpoints of death or disability and death with standard-dose rtPA in the included studies were comparable with the NINDS trial and SITS-MOST registry, respectively.
The observational studies in Asia employed variable doses of rtPA and reported conflicting findings for the functional outcome as well as for sICH. While a Taiwanese study of 1004 patients reported better outcomes with low-dose rtPA in patients with AIS aged 71–80 years,23 low-dose rtPA produced comparable results with the standard dose in a large observational registry from South Korea.26 In contrast, the Chinese registry, Thrombolysis Implementation and Monitor of Acute Ischemic Stroke in China, showed that standard-dose rtPA produced more favourable outcomes without increasing the risk of sICH when compared with low-dose regimens.29 The results from this uniformly Asian AIS registry were comparable with those from the multinational Safe Implementation of Thrombolysis in Stroke-Non-European Union World study.34
The prevalence of small vessel disease among Asian patients with AIS is relatively high.44 Furthermore, some racial differences exist in the thrombolytic effect of rtPA between Asian and Caucasians patients with AIS.45 Therefore, identification of the optimal dose of rtPA for Asian patients is important. The ENCHANTED trial was designed to compare the effects of low-dose rtPA with those of standard dose. The trial recruited eligible patients with AIS at 111 clinical centres in 13 countries worldwide.14 While the ENCHANTED trial was not able to confirm the non-inferiority of low-dose rtPA as compared with the standard-dose for death or disability (OR 1.09, 95% CI 0.95 to 1.25; P=0.51 for non-inferiority), it did demonstrate the non-inferiority for ordinal outcome of mRS (OR 1.00, 95% CI 0.89 to 1.13; P=0.04 for non-inferiority) and the fact that low-dose rtPA reduces the risk of sICH (SITS-MOST: OR 0.47, 95% CI 0.27 to 0.80; P=0.01). There was no heterogeneity of treatment effect between Asians and non-Asians. Interestingly, the pooled estimate of the ENCHANTED trial and observational studies failed to show the latter relationship. Although difficult to substantiate, this could have occurred due to the different criteria for defining sICH across the observational studies. Another possible reason that could have influenced the results is the rtPA bolus dose. Marketing authorisation in non-Asian countries recommended an rtPA dose of 0.9 mg/kg (not to exceed 90 mg total dose) infused over 60 min, with 10% of the total dose administered as an initial bolus over 1 min.46 Although the ENCHANTED trial adopted low-dose (0.6 mg/kg) rtPA as one treatment arm, the bolus dose was 15% of the total dose (mean: 6.2 mg) so that it was comparable with the amount received by the subjects recruited in the standard-dose group (mean: 6.3 mg). It is important to note that the rationale for increasing the bolus dose in the low-dose arm was to balance the chances of arterial recanalisation induced by the rtPA bolus during the first 23 min.47 The effect of the rtPA bolus dose could not be analysed since this information was missing in most of the observational studies.
There are several limitations in this review. First, the comparison of results between studies is difficult due to different baseline characteristics, differences in local practices and expertise, and various doses adopted. Second, most of the studies included a fairly small number of subjects, together with potential bias arising from the non-randomised nature of observational studies. This bias cannot be compensated for satisfactorily, and the unadjusted results from observational studies remain less conclusive even when the data from a large randomised controlled trial are combined. Third, different types of ischaemic stroke and varied stroke severity, with different responses to tissue plasminogen activator and different risk of sICH, were included in the studies, which may also contribute to the negative results. Lastly, there is evidence that the bolus dose differed widely among various observational studies and contributed to our results.47 48
In conclusion, our review shows a small difference between the outcomes or the risk profile in the studies using low-dose and/or standard-dose rtPA for AIS. Low-dose rtPA was not associated with lower risk of death or disability, death alone, or sICH.
Footnotes
Contributors XW, VKS and JC conceived the study. XW, SY and DZ were involved in the article screening process and data extraction. All authors were involved in drafting of the manuscript and in critically reviewing and revising it. All authors provided final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately addressed.
Funding The work was supported by grants from the National Natural Science Foundation of China (81471199) and the Department of Science and Technology of Jiangsu Province (BK20161113).
Competing interests TR is a National Institute for Health Research Senior Investigator, and reports receiving speaking fees from Bayer and Boehringer Ingelheim, and fees for Advisory Panels from Bayer and Daiichi Sankyo. CSA reports receiving fees for Advisory Panels of AstraZeneca and Medtronic, speaking at seminars for Takeda China and Boehringer Ingelheim, and a research grant from Takeda China. JC reports research grants and lecture fees from Servier for the ADVANCE trial and post-trial follow-up.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/