Rationale and Study Design to Assess the Efficacy and Safety of Minocycline in Patients with Moderate to Severe Acute Ischaemic Stroke (EMPHASIS)
- Yao Lu1,
- Ling Guan1,2,3,
- Meiyang Zhang1,
- Qianqian Yang1,
- Baoshan Qiu1,4,
- Dongyang Zhou1,5,
- Yicong Wang1,
- Yuesong Pan1,3,
- Luyan Wang1,3,
- Xuejiao Zhou1,
- Hui Qu1,3,
- Xiaoling Liao1,3,
- Liping Liu1,3,
- Xingquan Zhao1,3,
- Philip M Bath6,
- S Claiborne Johnston7,
- Pierre Amarenco8,9,
- Yongjun Wang1,3,
- Yilong Wang1,3
- 1Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2The University of British Columbia Department of Medicine, Vancouver, British Columbia, Canada
- 3China National Clinical Research Center for Neurological Diseases, Beijing, China
- 4Chinese Institute for Brain Research, Beijing, China
- 5Department of Neurology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 6Stroke Trials Unit, Mental Health & Clinical Neuroscience, University of Nottingham, Nottingham, UK
- 7Dell Medical School, The University of Texas at Austin, Austin, Texas, USA
- 8Department of Neurology and Stroke Center, University Paris Cite, Paris, France
- 9Population Health Research Institute, McMaster University, Hamilton, New Zealand, Canada
- Correspondence to Professor Yilong Wang; yilong528{at}aliyun.com
- Received 25 July 2024
- Accepted 21 November 2024
Abstract
Background Inflammation and blood-brain barrier disruption may contribute to the pathogenesis of ischaemic stroke. Minocycline was shown to exert anti-inflammatory effects by attenuating microglial activation and protecting blood-brain barrier in preclinical studies. Previous small-scale clinical studies have suggested that minocycline may have a potential beneficial effect on prognosis in acute ischaemic stroke. However, the efficacy and safety of minocycline in patients with acute ischaemic stroke need to be further confirmed.
Study aims We designed the study, Efficacy and Safety of Minocycline in Patients with Moderate to Severe Acute Ischaemic Stroke (EMPHASIS), to evaluate the effect of minocycline in improving the functional outcome and the drug safety in patients with acute ischaemic stroke.
Methods The EMPHASIS study is a multicentre, randomised, double-blind, placebo-controlled trial aiming to recruit patients with acute ischaemic stroke. Patients who had ischaemic stroke within 72 hours of onset, a National Institutes of Health Stroke Scale score between 4 and 25 and Ia≤1 (moderate-to-severe) will be randomly allocated to either minocycline or placebo groups in a 1:1 ratio. Patients will receive minocycline (or placebo) with a loading dose of 200 mg, and subsequent 100 mg every 12 hours for 4 days. All patients will receive routine guideline-based treatment. The primary efficacy outcome is an excellent functional outcome assessed by the proportion of modified Rankin Scale score of 0–1 at 90±7 days. The main safety outcomes include the number of symptomatic intracranial haemorrhage at 24±2 hours and 6±1 days.
Discussion The EMPHASIS trial is the first phase III trial to investigate whether minocycline is effective and safe in improving functional outcome at 90 days in patients with moderate-to-severe acute ischaemic stroke. The data generated may provide valuable evidence of a potential anti-inflammation treatment for ischaemic stroke.
- Ischemic Stroke
- Inflammation
- Clinical Trial
WHAT IS ALREADY KNOWN ON THIS TOPIC
Minocycline has an anti-inflammatory effect from attenuating microglial activation and maintaining the integrity of blood-brain barrier in preclinical studies. Previous small-scale clinical studies have also indicated that minocycline may have a positive impact on prognosis in acute ischaemic stroke. Further validation of its efficacy and safety is warranted through a large-scale randomised controlled trial.
WHAT THIS STUDY ADDS
The Efficacy and Safety of Minocycline in Patients with Moderate to Severe Acute Ischaemic Stroke (EMPHASIS) trial will address the issue of whether minocycline is effective and safe in patients with an acute ischaemic stroke (National Institutes of Health Stroke Scale between 4 and 25 and Ia≤1) within the past 72 hours.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
EMPHASIS study will provide valuable evidence for the potential use of minocycline as a complementary therapy to the routine treatment of acute ischaemic stroke.
Introduction and rational
Acute ischaemic stroke (AIS) is a major contributor to global mortality and disability, of which the key treatment is vascular recanalisation to improve the perfusion of ischaemic penumbra and prevent the progression of ischaemic injury.1–3
However, owing to the narrow therapeutic time window, strict patient selection and technical requirements, only a small subset of patients can successfully receive reperfusion treatment and restore cerebral perfusion.4 5 There is a pressing necessity to develop novel neuroprotective strategies to further reduce disability in these patients.
Neuroinflammation may be one of the mechanisms initiating brain tissue damage related to AIS. Within a few hours of AIS, injured cells release ‘danger signals’, such as damage-associated molecular patterns and activate microglia. Activated microglia secrete proinflammatory cytokines, damage the blood-brain barrier (BBB) through the matrix metalloproteinase (MMP) pathway and recruit peripheral immune cells, triggering a cascade of local and global inflammatory reactions in the brain and leading to long-term neurological deficits.6–8 Therefore, in patients with AIS, early regulation of neuroinflammation to further improve neuronal survival may be an effective strategy worth exploring in the future.
Minocycline is a second-generation semisynthetic tetracycline that has been widely used for the treatment of skin and other infections.9 When taken orally, it has good pharmacokinetic characteristics, including high bioavailability, a long half-life and high safety for long-term application. Moreover, its high lipophilicity allows it to readily penetrate the BBB and play multiple roles in regulating neuroinflammation: anti-inflammation, anti-apoptosis and anti-oxidation.10 11
In preclinical experiments, minocycline can inhibit factors such as MMP-2 and MMP-9,12 13 mitigating ischaemia-reperfusion injury, enhancing neuronal survival and supporting BBB remodelling.14 It also functions as an anti-inflammatory agent by suppressing the proliferation and activation of microglia15 16 as well as shifting proinflammatory microglia towards anti-inflammatory phenotype.17 Therefore, minocycline may serve as a promising therapeutic agent with multiple targets and mechanisms to treat patients with AIS.
A meta-analysis including four human studies and 14 rodent experiments showed that receiving minocycline may have effect on reducing National Institutes of Health Stroke Scale (NIHSS) score, modified Rankin Scale (mRS) score and infarct volume in acute ischemic stroke.18 Another systematic review involving 35 animal studies and six clinical trials explored the neuroprotective effect of minocycline against ischaemia-induced damage, and revealed that minocycline may increase the activity of neurons and reduce the infarct volume.19
Regarding its safety, there is no evidence that minocycline might increase the risk of intracranial haemorrhage in ischaemic stroke, but previous studies contained small sample sizes, which may limit the study conclusion.20–22 Besides, the possible effect of minocycline on BBB and platelet-related inflammation might have an effect on the risk of intracranial haemorrhage.
Therefore, we designed this phase III clinical trial to prove the efficacy and safety of minocycline. We hypothesise that minocycline may reduce the excess neuroinflammation during AIS and improve the clinical outcomes.
Methods
Study design
Efficacy and Safety of Minocycline in Patients with Moderate to Severe Acute Ischaemic Stroke (EMPHASIS) is a prospective, multicentre, randomised, double-blind, placebo-controlled parallel trial. Eligible participants will be randomly allocated to one of the two treatment groups receiving either minocycline or placebo in a ratio of 1:1, with a follow-up duration of 90 days. The study will be conducted at 58 hospitals in China (online supplemental table 1).
Supplementary data
Study population
The study will recruit patients with moderate-to-severe AIS. Participants are eligible if they are aged 18–80 years, suffered AIS in the past 72 hours with an NIHSS score between 4 and 25 and Ia≤1 and have functional independency assessed by mRS≤1 before the current attack. The main exclusion criteria for participating in the study include being allergic to tetracycline antibiotics or any component of minocycline, and use of tetracycline antibiotics within one week. The detailed inclusion and exclusion criteria are shown in box 1.
Inclusion and exclusion criteria of Efficacy and Safety of Minocycline in Patients with Moderate to Severe Acute Ischaemic Stroke trial
Inclusion criteria
Aged between 18 and 80 years.
Having acute ischaemic stroke (diagnosed by CT or MRI) in the past 72 hours.
Having 4≤NIHSS score ≤25, and the level of consciousness level (Ia)≤1.
The first stroke, or mRS score ≤1 prior to this episode.
Informed consent signed by the patient or their legal representative.
Exclusion criteria
Having a history of pseudomembranous colitis or antibiotic-related colitis.
Being allergic to tetracycline antibiotics or any component of the study drug.
Being tolerant to tetracyclines.
Taking tetracycline antibiotics in the past 7 days.
Having a community-acquired bacterial infection, for example, urinary tract infection or pneumonia.
Having a history of intracranial haemorrhage in the past 3 months, for example, parenchymal haemorrhage, intraventricular haemorrhage, subarachnoid haemorrhage, subdural or external haematoma.
Having brain tumours or vascular malformations.
Having large vessel occlusion with uncommon or undetermined aetiology, for example, vasculitis and arterial dissection.
Having severe renal insufficiency (blood creatinine >3.0 mg/dL (265.2 µmol/L), or glomerular filtration rate <30 mL/min/1.73 m2, or receiving dialysis), or having severe hepatic insufficiency (serum ALT or AST >three times of the upper limit of normal).
Having bleeding tendency, including receiving heparin in the past 48 hours with APTT≥35 s, receiving oral warfarin with INR>2, having platelet count <100×109/L, or having inherited bleeding disorders.
Receiving tretinoin, androgen or antiandrogen treatment (eg, anabolic steroids, spironolactone) in the past 3 months.
Having a history of intracranial or spinal surgery in the past 3 months; having a history of surgery or severe trauma in 1 month.
Pregnant, likely pregnant or breastfeeding.
Life expectancy less than 6 months.
Participated in any other clinical trial in the past 3 months.
Having any conditions that, in the opinion of the clinician may represent difficulties for the subjects’ full participation in the study, such as unable to follow the study procedures due to physical, cognitive, emotional or mental disorders.
ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate transaminase; INR, international normalised ratio; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Blinding and randomisation
This study employs a double-blind design. To ensure blinding, the treatment and control drugs are uniformly packaged, with each package bearing a unique random number. Subjects’ medications are pre-allocated according to the random code table. Randomisation allocation will be generated by separate statistical teams using a centralised computer. To maintain an approximate balance of allocation in a 1:1 ratio, block randomisation with fixed block sizes is adopted. For participants eligible for randomisation, each subcentre will assign a random number in an ascending sequence. Unblinding is permitted when adverse events occur and disclosing investigational drug information is essential for the participant’s treatment.
Intervention
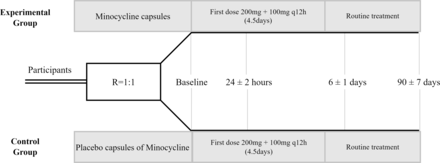
Eligible participants will be randomly allocated into minocycline or placebo groups in a 1:1 ratio. All participants will receive an initial dose of 200 mg within 30 min after randomisation and followed by 100 mg every 12 hours for 4 days (figure 1). Minocycline hydrochloride or placebo capsules will be administered orally or through a nasogastric feeding tube if the participant has dysphagia. All study intervention is based on participants’ routine guideline-based treatment.
The flow chart of Efficacy and Safety of Minocycline in Patients with Moderate to Severe Acute Ischaemic Stroke trial.
Follow-up visits and data collection
All study patients are hospitalised. We will visit participants at baseline, 24±2 hours, 6±1 days and 90±7 days.
At baseline, the study will record details of the presenting stroke, medical history, medication use, physical examination, mRS before stroke onset, NIHSS score, laboratory biomarkers including high-sensitivity C-reactive protein (hs-CRP) level. 6 mL of venous blood will be collected for the assessment of inflammation and BBB-related biomarkers. The neuroimaging data used to diagnose ischaemic stroke will also be retrieved.
At 24±2 hours and 6±1 days, researchers will evaluate the NIHSS score and re-collect the blood sample. hs-CRP will be repeatedly assessed at 6±1 days.
At 90±7 days, the mRS score will be evaluated by a trained local investigator by telephone or in-person interview. The process of evaluation should be recorded and the mRS score is centrally corrected by clinical inspectors to ensure the accuracy and consistency. Other variables include EuroQol Group 5-Dimension (EQ-5D) questionnaire score and adverse events (table 1).
All adverse events will be recorded including the date and possible causes. Participants are encouraged to contact their local centre to report any symptom occurring during the follow-up visits. Collected and verified data will be transmitted using the electronic data capture system.
Assessment schedule
Study outcomes
Efficacy outcome
The primary outcome is an excellent functional outcome measured by the proportion of the mRS score of 0–1 at 90±7 days. Secondary outcomes include mRS distribution at 90±7 days; (2) changes in NIHSS score at 24±2 hours and 6±1 days compared with baseline score; (3) changes in hs-CRP level at 6±1 days compared with baseline level; (4) early neurological deterioration at 24±2 hours and 6±1 days (5) recurrent stroke (including ischaemic and haemorrhagic stroke) at 90±7 days; (6) recurrent ischaemic stroke at 90±7 days; (7) composite vascular events (stroke, myocardial infarction and vascular death) at 90±7 days; (8) quality of life (EQ-5D) score at 90±7 days.
Exploratory outcomes include changes in the levels of venous neuroinflammation and thrombo-inflammation indicators at 24±2 hours and 6±1 days compared with baseline levels; cerebral haemodynamic function at 6±1 days and 90±7 days as well as changes in the levels of venous intestinal flora metabolites (plasma TMAO [trimethylamine N-oxide] and its precursors, etc) at 6±1 days compared with baseline levels.
Safety outcomes
The main safety outcome includes symptomatic intracranial haemorrhage at 24±2 hours and 6±1 days, including both spontaneous and treatment-related. Other safety outcomes include any bleeding event classified by Global Utilisation of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries Criteria,23 vascular death, all-cause death, investigator-reported adverse events/serious adverse events at 90±7 days and antibiotic-associated diarrhoea, enteritis and constipation at 6±1 days.
The detailed definitions of study outcomes mentioned above are shown in online supplemental table 2.
Sample size estimate
We calculate the proportion of mRS of 0–1 at 3 months after the onset of initial acute ischaemic stroke in the China National Stroke Registry III. The result shows 60% of patients could reach an mRS 0–1 score at 3 months and we use it as the estimate of event rate in the control group. According to previous studies,24 we hypothesize that participants who receive minocycline therapy will increase 8% of the excellent functional outcome. Considering a drop-out rate of 10%, a total of 1672 patients (836 per group) are required to give 90% power to detect the difference of the proportion of mRS 0–1 between the minocycline group and the control group, under a two-tailed significance level of 0.05.
Statistical analyses
In this study, intention-to-treat analysis (ITT) will be used to compare study outcomes between treatment groups. For efficacy outcomes, Chi-squared test and generalized linear model will be used to evaluate the proportion of mRS 0–1 (the primary outcome) between minocycline and control groups in 90 days. Shift analysis of mRS score using an ordinal logistic regression model will also be analyzed. Kaplan-Meier curves and log-rank tests will be used to compare the cumulative risks of vascular events between two treatment groups at 90 days. Cox-proportional hazards regression and generalized linear model will be used to compare different secondary and exploratory efficacy outcomes, as well as the subgroup analyses (below). Confounding factors will be included in the multivariate models based on the result of univariate analysis, previous literature and clinical knowledge. To compare safety outcomes and AE/SAE between the two treatment groups, both Chi-squared test (or Cochran-Mantel-Haenszel test) and survival analysis will be used. A two-sided p value of <0.05 will be deemed statistically significant and all analyses will be performed using SAS software V.9.4 (SAS Institute).
Subgroup analyses will evaluate the different effects of minocycline according to age, gender, concomitant diseases (such as hypertension, hyperglycaemia, hyperlipidaemia, previous ischaemic stroke), smoking, drinking, aetiological classification and disease severity, type of therapy, time-to-treatment, the level of inflammatory or BBB-related biomarkers, cerebral haemodynamics, whether combing with cerebral small vessel diseases, intracranial and extracranial vessel status and other neuroimaging features of research interest.
Data safety monitoring and data organisation
The steering committee will provide scientific guidance and ensure the quality of the research. The executive committee will collect available blinded data, assess the trial progress and provide appropriate guidance for the study. The Data and Safety Monitoring Board (DSMB), consisted of independent academic experts and statisticians not involved in the trial, will monitor the trial progress periodically to ensure that the trial meets the standards of ethics and patient safety. DSMB charter will be certified by its members and the executive committee before enrolment. The clinical event adjudication committee, consisting of independent experts, will adjudicate outcome events uniformly (online supplemental table 3).
Patient data will be recorded and archived in the case record form (CRF). All data will be transmitted to the electronic CRF (eCRF, https://www.91trial.com) with password required for access. The data transfer between eCRF and CRF database will use an encrypted connection. The clinical investigators will check the completeness of eCRF, adherence to the study protocol and the consistency between eCRF and the original CRF data set. The principal investigators of all subcentres should be certified in Good Clinical Practice, and they are responsible for the data quality of their centres.
Discussion
Neuroprotection following AIS represents a promising therapeutic approach yet requires additional investigation due to inconsistent findings observed in previous clinical trials.25 The EMPHASIS trial is a multicentre, randomised, double-blind, placebo-controlled trial addressing the question of whether minocycline can enhance the functional prognosis of patients with AIS compared with placebo.
The information from prior clinical studies investigating the application of minocycline in patients with AIS is presented in online supplemental table 4. The first clinical study investigating minocycline in AIS randomly assigned 152 patients within 6–24 hours after onset to receive either oral minocycline or placebo at a dosage of 200 mg for 5 days. The results indicated a significant enhancement in outcomes for patients treated with minocycline compared with those receiving the placebo.26 Similar favourable effects were also observed in subsequent trails, indicating that timely and short-term administration of minocycline within a therapeutic window of 24 hours after AIS onset and lasting 3–5 days could be helpful in reducing neurological deficits.24 27 28 However, a pilot study suggested that commencing five doses of intravenous minocycline within 24 hours following stroke onset was safe but lacked efficacy.29 Previous clinical trials assessing minocycline in AIS exhibited substantial heterogeneity and were conducted with open-label or non-randomised study design, which were underpowered to reliably ascertain the treatment effect of minocycline.
Several clinical studies on minocycline recruit patients with stroke who underwent reperfusion therapy, for example, the Effects of Minocycline on Patients With Ischaemic Stroke Undergoing Intravenous Thrombectomy (NCT05487417), Minocycline for Acute Ischaemic Stroke Undergoing Endovascular Treatment Due to Basilar Artery Occlusion (NCT05512910) and Minocycline Efficacy in Improving Neurological Outcome of Patients Who Undergo Endovascular Revascularisation for Acute Ischaemic Stroke (NCT05367362). These studies support the study rationale that minocycline may have an effect on anti-neuroinflammation and improve the clinical outcomes combined with reperfusion therapy of ischaemic stroke as a neuroprotectant. However, evidences have demonstrated that neuroinflammation plays an important role in all phases of ischaemic stroke.7 8 30 Several clinical studies thus designed to recruit participants with ischaemic stroke including both the ones receiving reperfusion therapy and others with medication, for instance, Perth Iv Minocycline Stroke Study (ACTRN12612000237886), Neuroprotection With Minocycline Therapy for Acute Stroke Recovery Trial (NCT00930020) and the ongoing open-label clinical trial ‘Maimonides Minocycline in Stroke Study (NCT06107725)’ which explores the efficacy of minocycline in acute stroke within 24 hours. In order to examine the effect of minocycline in the whole stage of ischaemic stroke and improve the study’s generalisability, we designed this study to include patients with ischaemic stroke irrespective of their treatment. In this phase-III clinical trial, we could verify the efficacy and safety of minocycline on all patents with ischaemic stroke and further compare the anti-neuroinflammatory effect of minocycline between participants who have medication therapy and those with futile recanalisation.
Our study has several strengths. First, the sample size surpasses that of previous studies, enhancing the precision of minocycline treatment effect analysis. Second, the trial employs a multicentre, double-blind and block-randomised design to mitigate potential confounders and prevent selection biases. Third, considering that microglial activation peaks at 1 week and shows minimal activation before 72 hours post-stroke in positron emission tomography scans,6 31 we extend the enrolment window to 72 hours, potentially broadening the population that may benefit from treatment. Lastly, in addition to assessing neurological function and life independence, we also longitudinally collect venous blood samples to monitor inflammatory biomarkers at baseline, 24 hours and 6 days providing valuable insight into dynamic post-stroke inflammatory response.
Summary and conclusion
EMPHASIS is the first prospective, multicentre, randomised, double-blind, placebo-controlled trial with the largest sample size to date to explore the effect of minocycline in patients with AIS. This trial aims to generate novel evidence elucidating the efficacy and safety of minocycline in patients with AIS.
Data availability statement
Data sharing not applicable as no data sets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
Ethics approval
This study was approved by the Institutional Review Board of Beijing Tiantan Hospital (KY2023-007-04), and all other participating study centres granted their approval for this trial. Before enrolment, participants provided informed consent to participate in the study.
Acknowledgments
We sincerely acknowledge all the patients and investigators participating in the trial.
Footnotes
YL and LG are joint first authors.
X @yilong
YL and LG contributed equally.
Contributors YLW had full access to all the study data and takes responsibility for the integrity and accuracy of the data. YL and LG contributed equally to this work as first authors. YLW and LG proposed the study conception and study design. YL and LG prepared the draft of the manuscript. YL, QQY and MYZ conduct the study and collect data. BSQ conducted the animal expriments and provided theoratical support on this study. HQ, XJZ, XLL and YCW help conduct the clinical trial and patient management. YSP and LYW prepare the statistical analysis plan and conduct statistical analyses. DYZ supports study conduction and data management. SCJ, PA, PMB, LPL, XQZ and YJW supervised the study design and edited the article. None of the authors received payment for writing the article. All authors approve the submission of this version of the manuscript.
Funding This work is supported by the following grants: (1) The National Natural Science Foundation of China (No. 81825007, 82271516); (2) Beijing Healthunion Cardio-cerebrovascular Disease Prevention and Treatment Foundation (No. 20230418-J-E-010).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.