Baseline systolic blood pressure and efficacy of dual antiplatelet in acute ischaemic stroke
- Correspondence to Professor Hui-Sheng Chen; chszh{at}aliyun.com
- Received 9 August 2024
- Accepted 21 November 2024
Abstract
Objective Systolic blood pressure (SBP) affects the risk of early neurological deterioration (END). This subgroup analysis of Antiplatelet Therapy in Acute Mild to Moderate Ischemic Stroke (ATAMIS) trial aimed to explore whether SBP at admission affected the efficacy of different antiplatelet therapies in preventing END.
Methods Based on the modified intention-to-treat analysis set of the ATAMIS trial, patients were divided into two subgroups according to whether SBP at admission was equal to or higher than 140 mm Hg, which were further subdivided into clopidogrel plus aspirin and aspirin alone treatments according to the randomised assignment. We conducted multivariable regression analyses to detect relationship between SBP at admission and END, as well as efficacy of different antiplatelet therapies in each SBP subgroup. Primary endpoint was END defined as ≥2-point increase in 7-day National Institutes of Health Stroke Scale score. Safety endpoints included intracranial haemorrhage and bleeding events during the trial.
Results This study included 2915 patients. Risk of END raised by 16% as SBP at admission increased by every 10 mm Hg (p<0.001). Clopidogrel plus aspirin resulted in significantly lower risk of END than aspirin alone in patients with SBP≥140 mm Hg (5.5% vs 7.9%; adjusted risk difference (RD) and 95% CI −2.5% (−4.1% to −1.0%)), but not in those with SBP<140 mm Hg (3.4% vs 4.2%; adjusted RD and 95% CI −0.8% (−3.2% to 1.7%)). Efficacy of different antiplatelet therapies and SBP did not show significant interaction (p=0.50). Safety endpoints were similar between treatments in SBP subgroups.
Conclusion The risk of END increases with elevated SBP at admission among patients with acute mild-to-moderate ischaemic stroke who are not suitable for reperfusion treatments. Fewer END occurred following clopidogrel plus aspirin compared with aspirin alone across different SBP levels. The finding should be interpreted cautiously.
- Stroke
- Blood Pressure
WHAT IS ALREADY KNOWN ON THIS TOPIC
Systolic blood pressure (SBP) affects the risk of early neurological deterioration following stroke and the efficacy of dual antiplatelet in preventing stroke recurrence. However, no study detected the relationship between SBP and efficacy of dual antiplatelet in reducing the risk of early neurological deterioration.
WHAT THIS STUDY ADDS
In patients diagnosed with acute mild-to-moderate ischaemic stroke but not suitable for reperfusion treatment, the risk of 7-day early neurological deterioration increased with elevated SBP at admission. Different from monotherapy with aspirin alone, dual antiplatelet with clopidogrel and aspirin resulted in a decreased risk of early neurological deterioration regardless of patients’ SBP at admission.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These findings support the use of dual antiplatelet with clopidogrel and aspirin for preventing the occurrence of early neurological deterioration in acute mild-to-moderate ischaemic stroke regardless of SBP at admission. This should be interpreted cautiously.
Introduction
Early neurological deterioration (END) was a serious complication and predicted poor functional outcomes following acute ischaemic stroke.1 In clinical practice, it remains important to find effective treatment to reduce the risk of END. Recent three randomised clinical trials demonstrated that stronger antithrombotic strategies may be effective to prevent the occurrence of END or improve the functional outcomes following END in acute ischaemic stroke.2–4 History of hypertension, higher blood pressure parameters at admission and blood pressure variability at acute phase were found to predict the occurrence of END.5–7 Chronic hypertension reduced the ability of collateral development by impairing microvascular function and change in blood pressure reduced cerebral haemodynamic reserve,8 which contributed to the development of END. Whether the blood pressure in acute phase will affect the efficacy of antithrombotic strategies is worth exploring.
Previous studies detected the relationship between baseline systolic blood pressure (SBP) level and the choice of initial antiplatelet therapy9 or efficacy of dual antiplatelet in preventing stroke recurrence.10 However, few studies investigate the relationship between baseline SBP levels and efficacy of dual antiplatelet therapy in preventing the occurrence of END. Dual antiplatelet therapy reduced the occurrence of END based on its strong antithrombotic efficacy, which may result in improving cerebral blood flow supply.11 Given that change in blood pressure reflected poor function in cerebral autoregulation caused by direct damage to the modulatory centres in acute ischaemic stroke (eg, an overall impaired autoregulation when cerebral perfusion pressure increased 30%),12 13 which was associated with neurological deterioration after stroke by affecting cerebral blood flow supply and perfusion pressure,14 15 the level of SBP at admission may be associated with the efficacy of dual antiplatelet in preventing the occurrence of END.
According to the Antiplatelet Therapy in Acute Mild to Moderate Ischemic Stroke (ATAMIS) trial, dual antiplatelet therapy with clopidogrel and aspirin, compared with aspirin alone, significantly prevented END at 7 days in patients diagnosed with acute mild-to-moderate ischaemic stroke who were not suitable for reperfusion treatments.4 Based on the above discussion, we performed a subgroup analysis of ATAMIS to determine the association of SBP at admission with efficacy of different antiplatelet therapies in this population.
Methods
Study design and population
Previous studies reported the details of ATAMIS trial,4 16 which was a randomised clinical trial designed with multicentre, open-label and blinded-endpoint, enrolling 3005 patients between 20 December 2016 and 9 August 2022, and finishing in October 2022. The trial aimed to compare the efficacy in preventing the occurrence of END between clopidogrel plus aspirin and aspirin alone in the target population, who were administrated within 48 hours of symptom onset and diagnosed with acute ischaemic stroke presenting 4–10 points on the National Institutes of Health Stroke Scale (NIHSS) score at admission. Patients were excluded if they received any reperfusion treatment, were treated with anticoagulation drugs, experienced intracerebral haemorrhage, urinary tract bleeding or gastrointestinal bleeding, received carotid revascularisation and were allergic to study drugs. Detailed information about the screening criteria and defining analysis set were shown in the previous report.4 The modified intention-to-treat analysis was conducted as the primary analysis strategy in the ATAMIS trial, from which all the patients were included in the current study. The current study was in accordance with the principle of the Consolidated Standards of Reporting Trials.
Patient and public involvement
No patients were involved.
Procedures
In the ATAMIS, patients enrolled were randomly assigned to receive clopidogrel plus aspirin (day 1: 300 mg clopidogrel and 100 mg aspirin; days 2–14: 75 mg clopidogrel and 100 mg aspirin) or aspirin alone (days 1–14: 100–300 mg aspirin).4 In this analysis, included patients were first divided into two subgroups: SBP≥140 mm Hg and SBP<140 mm Hg based on the results from our previous study, which indicated that dual antiplatelet therapy significantly reduced the occurrence of END in patients with minor stroke and presenting ≥140 mm Hg at admission.17 The baseline SBP was measured at admission. In the ATAMIS trial, if the SBP≥200 mm Hg or diastolic blood pressure (DBP) ≥110 mm Hg, patients were treated with antihypertensive treatment to control blood pressure according to the current guideline in China. In the visits of admission, day 7 and day 14 after randomisation, NIHSS score was measured to evaluate the neurological status. In the follow-up visit of day 90 after randomisation, modified Rankin Scale (mRS) score, bleeding or ischaemic vascular events were collected.
Outcomes
In the current study, all the endpoints were kept consistent with the ATAMIS.4 The primary endpoint was END, defined as ≥a 2-point increase in 7-day NIHSS score compared with admission (excluding due to cerebral haemorrhage).18 The secondary endpoints included excellent functional outcome (mRS scoring 0–1 at 90 days); shift distribution of ordinal mRS scores at 90 days; change in 14-day NIHSS scores compared with admission; time from randomisation to the occurrence of new stroke19; and time from randomisation to the occurrence of other vascular events or all-cause mortality. The safety endpoints were set as any bleeding events and intracranial haemorrhage which may result from antiplatelet treatments.
Statistical analysis
Adjusted analysis was defined as the primary analysis to remove the potential imbalanced bias from characteristics between treatment groups in post hoc analysis. We summarised categorical variables as frequencies with percentages and continuous variables as median with IQR for the baseline characteristics, which were compared by χ2 test and Mann-Whiney U test. We calculated the absolute number of events for the endpoints, and estimated the treatment effects with 95% CIs using risk difference (RD) for END, excellent functional outcome and safety endpoints, OR for ordinal mRS scores, geometric mean ratio for change in NIHSS scores or HR for time-dependent endpoints, which were parallel with the ATAMIS trial.4
First, as a continuous variable, binary logistic regression analysis was performed to explore the association of END with SBP at admission. Multivariable logistic regression model was performed to calculate probability score by including covariates such as antiplatelet therapy, age, sex, diabetes mellitus, history of hypertension, time from stroke onset to antiplatelet therapy, NIHSS score at randomisation and presumed stroke cause,20 which were prespecified in the ATAMIS trial.4
Second, efficacies of different antiplatelet therapies were investigated by adjusted and unadjusted generalised linear models or Cox regression models in dichotomous SBP subgroups. Unbalanced baseline variables compared between treatment groups with p<0.1 were included in the adjusted model. Interactions between SBP and efficacy of antiplatelet therapy were conducted by adjusting unbalanced baseline variables between SBP subgroups.
Third, considering the unbalanced sample size between SBP subgroups, we performed propensity score matching analysis to address the bias. Baseline variables with p<0.1 between subgroups and treatments were matched by the nearest method, 1:1 of ratio and 0.05 of calliper value. The risk of END between treatments will be compared in each SBP subgroup of the new analysis set.
Exploratory analyses were performed in the current study and evaluated the nominal p value that would be statistically significant if it was less than 0.05. Analyses were conducted by SPSS software (V.26.0) and figures were prepared by R software (V.4.1.3, R Foundation for Statistical Computing).
Results
Patient characteristics
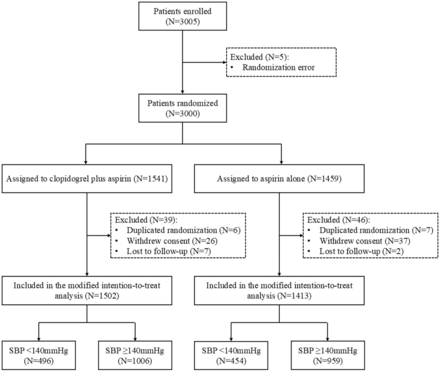
Overall, 2915 patients were included in the current study including 1502 patients assigned to clopidogrel plus aspirin (454 patients with SBP<140 mm Hg and 959 patients with SBP≥140 mm Hg) and 1413 patients assigned to aspirin alone (496 patients with SBP<140 mm Hg and 1006 patients with SBP≥140 mm Hg). The flow diagram of patient screening is shown in figure 1.
Trial profile. SBP, systolic blood pressure.
In patients with SBP<140 mm Hg, there was not any significant imbalance of baseline characteristics between antiplatelet treatments. In patients with SBP≥140 mm Hg, there were some imbalances in diabetes mellitus, location of responsible vessel and DBP at admission between treatment groups. Between patients with SBP<140 mm Hg and those with SBP≥140 mm Hg, there were some imbalances in sex, history of hypertension, previous stroke, blood pressures at admission, blood glucose at admission, NIHSS score at admission, time from stroke onset to antiplatelet therapy and previous hypertension treatment. Further information about baseline characteristics between SBP subgroups or antiplatelet treatments is shown in table 1.
Baseline patient characteristics in the analysis
Association between baseline SBP and END
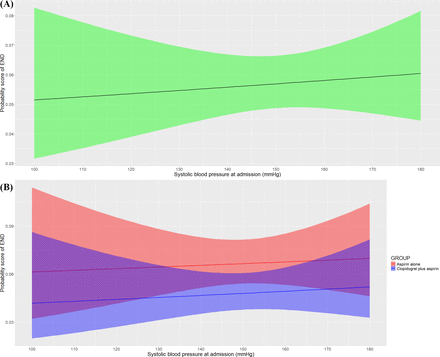
We investigated the relationship between continuous SBP at admission and risk of END. The risk of END was raised as the SBP at admission increased (figure 2A), which was also, respectively, detected in patients treated with different antiplatelet treatments (figure 2B). The risk of END increased as the SBP at admission increased in both the two treatment groups, kept higher in the aspirin alone group and the gap of risk between treatment groups seemed consistent as the SBP at admission increased. In the multivariable logistic regression analysis, the likelihood of END at 7 days was associated with SBP at admission (adjusted OR 1.16; 95% CI 1.08 to 1.24; p<0.001; figure 2), which indicated that the risk of END increased by 15.5% as every 10 mm Hg SBP at admission increased.
Association of baseline SBP with risk of END. (A) The probability score of END at 7 days raised with SBP at admission increasing. (B) The probability score of END was stratified by treatment groups, raised as the SBP at admission increased in both two treatment groups and kept higher in the aspirin alone group, and the gap of probability score between treatment groups seemed to be consistent. The baseline SBP was associated with the risk of END (adjusted OR, 1.16; 95% CI, 1.08 to 1.24; p<0.001). END, early neurological deterioration; SBP, systolic blood pressure.
Association between baseline SBP and efficacy of antiplatelet therapy
In table 2, the results showed clopidogrel plus aspirin significantly decreased risk of END compared with aspirin alone in patients with SBP≥140 mm Hg (5.5% vs 7.9%; adjusted RD and 95% CI −2.5% (−4.1% to −1.0%), p<0.01) but did not show a significant difference in the patients with SBP<140 mm Hg (3.4% vs 4.2%; adjusted RD and 95% CI −0.8% (−3.2% to 1.7%), p=0.54). Efficacy of different antiplatelet therapies and SBP did not show significant interaction (adjusted p=0.50). In the propensity score matching analysis, clopidogrel plus aspirin also resulted in fewer END than aspirin alone in each SBP subgroup. However, the difference between treatments in the SBP≥140 mm Hg subgroup lost statistical significance (table 3).
Association of categorical baseline systolic blood pressure with outcomes
Propensity score matching analysis for primary outcome
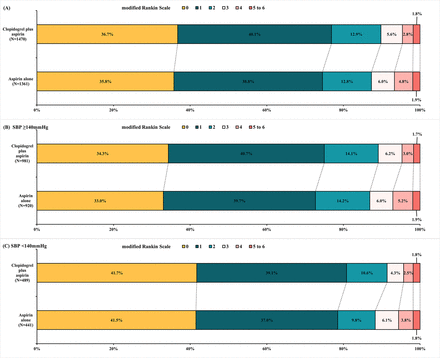
The 90-day mRS score distribution between antiplatelet treatments in SBP subgroups is shown in figure 3. The proportion of mRS scoring 0–1 between the two treatment groups was similar in the patients with SBP<140 mm (80.8% vs 78.5%; adjusted RD and 95% CI 2.3% (−2.9% to 7.5%), p=0.38) and the patients with SBP≥140 mm Hg (74.9% vs 72.7%; adjusted RD and 95% CI 2.2% (−0.6% to 5.0%), p=0.12). Furthermore, the ordinal regression analysis showed that the distribution of mRS score was also similar between two antiplatelet treatments in the patients with SBP<140 mm Hg (adjusted OR and 95% CI 1.07 (0.84 to 1.36), p=0.59) and the patients with SBP≥140 mm Hg (adjusted OR and 95% CI 1.09 (0.97 to 1.23), p=0.14). All the other secondary endpoints and safety endpoints were not significantly different between treatments in any subgroup, and no interactions were found (table 2).
Distribution of modified Rankin Scale Score at 90 days. The raw distribution of scores was shown in two treatment groups of all the patients (A), the patients with SBP≥140 mm Hg (B), and the patients with SBP<140 mm Hg (C). Scores ranged from 0 to 6. 0=no symptoms, 1=symptoms without clinically significant disability, 2=slight disability, 3=moderate disability, 4=moderately severe disability, 5=severe disability and 6=death. SBP, systolic blood pressure.
Discussion
In this ATAMIS post hoc analysis, we found that the risk of END increased as the SBP at admission increased in patients receiving dual-antiplatelet and mono-antiplatelet treatments. As we divided patients into SBP subgroups using 140 mmHg as a cut-off, we found that clopidogrel plus aspirin resulted in fewer END compared with aspirin alone in those with baseline SBP≥140 mmHg. However, the difference lost significance after balancing sample size between SBP subgroups.
END was previously reported to be a serious and frequent complication following stroke.21 Although blood pressure is an important clinical characteristic of patients diagnosed with ischaemic stroke and contributes to patients’ prognosis,22–24 the association between baseline SBP levels and END remains unclear.5 In the current study, we found that the risk of END raised with elevated SBP level at admission, which was similar to those reported in retrospective observational studies from China.25 It has previously been reported that higher SBP at admission reflected poor cerebral autoregulation,26 which was associated with poor prognosis after stroke by leading to great blood pressure variability.7 We found that the association of SBP at admission with END was consistent among patients treated with either dual antiplatelet therapy or antiplatelet monotherapy. However, in the current study, patients with SBP≥140 mm Hg at admission benefited more from dual antiplatelet therapy than antiplatelet monotherapy with respect to the occurrence of END. Increase in SBP following stroke might reflect the impairment of cerebral autoregulation function caused by direct damage to the modulatory centres after stroke, which was common in acute ischaemic stroke.12 13 Cerebral autoregulation maintained cerebral blood flow supply and perfusion pressure that was associated with stroke progression.14 15 Dual antiplatelet therapy with stronger antithrombotic effect contributed to preventing reocclusion of the responsible blood vessel, which would improve the poor cerebral blood flow supply caused by the impaired cerebral autoregulation.27 Additionally, increased cerebral blood flow was previously reported to result in improving neurological function.28 Thus, the effect of dual-antiplatelet versus mono-antiplatelet therapy on preventing END and improving neurological function among patients with SBP≥140 mm Hg may result from stronger antithrombotic effects of dual antiplatelet therapy, which may result in improvements in cerebral blood flow in the context of elevated blood pressure. Given that the significant difference in END between treatments disappeared after reducing sample size of patients with SBP≥140 mm Hg, the potential benefit in this population warrants further investigation. In addition, previous studies mainly investigated the association between blood pressure at admission and END with early time window from 24 to 72 hours.17 29 Considering that more confounders may affect the neurological deterioration with the time window expanding,30 the association may be weakened. Thus, the inference needs further validation by measuring cerebral blood flow and perfusion in the early stage following stroke and adopting END defined within shorter time window after stroke onset.
For the secondary outcomes, compared with aspirin alone, dual antiplatelet therapy with clopidogrel plus aspirin did not show a significant difference with respect to long-term functional outcomes such as mRS scoring 0 to 1 and distribution of mRS score at 90 days, which was similar to that from the Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) study.31 We inferred that this result may result from the mild neurological deficits of the population (median NIHSS scoring 4–5 at admission). In addition, lack of statistical power due to the original design of ATAMIS trial for preventing END and small sample size after dividing into SBP subgroups resulted in the similar occurrence of new stroke or other vascular events within 90 days between treatment groups.
We admitted several limitations in the current study. First, although a relatively large population with 950 and 1965 patients were, respectively, included in each SBP subgroup in the study, an unbalanced sample size may introduce bias. The propensity score matching analysis potentially addressed the selection and unbalanced bias. Second, due to the lack of blood pressure recorded within 24 hours following admission, we neither evaluated whether antihypertension treatment effectively controlled increased blood pressure nor explored the blood pressure variability in the first 24 hours which was obviously associated with the risk of END. Additionally, we also could not investigate whether elevated baseline blood pressure may maintain cerebral perfusion in the impaired areas as the level of blood pressure before this index ischaemic stroke was not available. Third, the findings lacked some examination of cerebral autoregulation such as cerebral blood flow or perfusion in the acute phase following stroke, which might support the inference about the association between baseline SBP and efficacy of dual antiplatelet therapy on improving neurological function. Fourth, as the ATAMIS did not include patients eligible for reperfusion treatments, as well as those with cardioembolic stroke, our findings are not generalisable to the above population. Fifth, a cohort with non-Chinese population would be needed to validate the generalisability. Finally, as the exploration characteristic of post hoc analysis, we cautiously interpreted our findings.
In conclusion, this ATAMIS subgroup analysis suggests that among patients who were diagnosed with acute mild-to-moderate ischaemic stroke and not suitable for reperfusion treatments, the risk of END raised with elevated SBP at admission, and clopidogrel plus aspirin treatment may produce benefit across SBP levels with respect to preventing 7-day END. This finding should be cautiously interpreted and needs to be confirmed in the future.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and all study procedures were reviewed and approved by the Ethics Committee of General Hospital of Northern Theater Command (Approval Number: k (2016) 6-1). Participants gave informed consent to participate in the study before taking part.
Footnotes
Contributors H-SC contributed to conception and design of the study; YC contributed to analysis of data and drafting the original manuscript; YW contributed to acquisition of data. H-SC is the guarantor.
Funding This study was funded by Science and Technology Project Plan of Liaoning Province (2019JH2/10300027).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.